polymorphism → polimorfija
Polymorphism is the ability of a solid substance to crystallise into more than one different crystal structure. Different polymorphs have different arrangements of atoms within the unit cell, and this can have a profound effect on the properties of the final crystallised compound. The change that takes place between crystal structures of the same chemical compound is called polymorphic transformation.
The set of unique crystal structures a given compound may form are called polymorphs. Calcium carbonate is dimorphous (two forms), crystallizing as calcite or aragonite. Titanium dioxide is trimorphous; its three forms are brookite, anatase, and rutile. The prevailing crystal structure depends on both the temperature and the external pressure.
Iron is a metal with polymorphism structure. Each structure stable in the range of temperature, for example, when iron crystallizes at 1 538 °C it is bcc (δ-iron), at 1 394 °C the structure changes to fcc (γ-iron or austenite), and at 912 °C it again becomes bcc (α-iron or ferrite).
Polymorphism of an element is called allotropy.
polypeptide → polipeptid
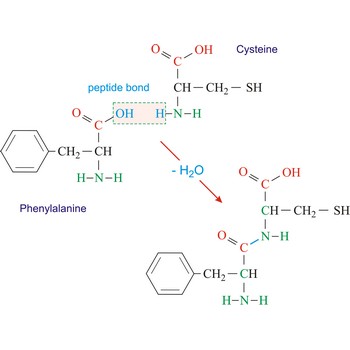
Polypeptides are peptides containing ten or more amino acid residues. The properties of a polypeptide are determined by the type and sequence of its constituent amino acids.
polysaccharide → polisaharid
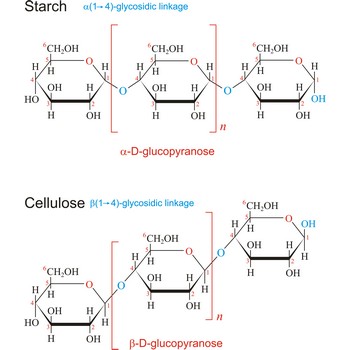
Polysaccharides are compounds consisting of a large number of simple sugars (monosaccharides) linked together by glycosidic bonds. When polysaccharides are composed of a single monosaccharide building block, they are termed homopolysaccharides. Heteropolysaccharides contain two or more different types of monosaccharide. Polysaccharides may have molecular weights of up to several million and are often highly branched. Since they have only the one free anomeric -OH group at the end of a very long chain, polysaccharides aren’t reducing sugars and don’t show noticeable mutarotation. The most common polysaccharides are cellulose, starch, and glycogen.
polyvinyl chloride → polivinil klorid
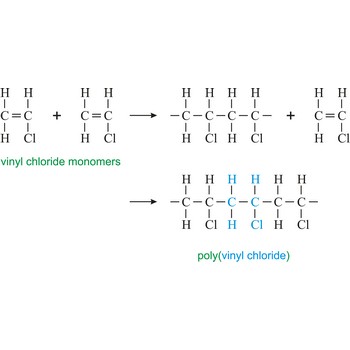
Poly(vinyl chloride) or the PVC is hard and resistant homopolymer produced by the polymerization of the gas vinyl chloride [CH2CHCl]. The pure polymer is hard, brittle and difficult to process, but it becomes flexible when plasticizers are added. After mixing with plasticizers, stabilizers, and pigments, the resin may be fabricated by techniques such as calendering, molding, or extrusion into flexible articles such as raincoats, shower curtains, and packaging films. The resin is not plasticized for use in making rigid products such as water pipe, plumbing fittings, and phonograph records.
potassium → kalij
Potassium was discovered by Sir Humphry Davy (England) in 1807. The origin of the name comes from the Arabic word qali meaning alkali (the origin of the symbol K comes from the Latin word kalium). It is soft, waxy, silver-white metal. Fresh surface has silvery sheen. Quickly forms dull oxide coating on exposure to air. Reacts strongly with water. Reacts with water to give off flammable gas. Reacts violently with oxidants. Occurs only in compounds. Potassium is found in minerals like carnallite [(KMgCl3)·6H2O] and sylvite (KCL). Pure metal is produced by the reaction of hot potassium chloride and sodium vapours in a special retort. Used as potash in making glass and soap. Also as saltpetre, potassium nitrate (KNO3) to make explosives and to colour fireworks in mauve. Vital to function of nerve and muscle tissues.
potential energy → potencijalna energija
Potential energy (Ep) is the energy stored in a body or system as a consequence of its position, shape, or state (this includes gravitation energy, electrical energy, nuclear energy, and chemical energy). Gravitational potential energy is the energy associated with the state of separation between bodies that attracts each other via gravitational force. Elastic potential energy is the energy associated with the state of compression or extension of an elastic object. Thermal energy is associated with the random motions of atoms and molecules in a body.
practical salinity → praktični salinitet
Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution). When K15 = 1, the Practical Salinity P S is by definition 35. The conductivity of that reference solution is C(35,1568,0) = 42.914 mS/cm = 4.2914 S/m (Siemens per meter). Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". When K15 is not unity, SP and K15 are related by the PSS-78 equation
At a temperature of t68 = 15 °C, Rt is simply K15 and Practical Salinity SP can be determined from the above equation. For temperatures other than t68 = 15 °C, Practical Salinity SP is given by the following function of Rt (k = 0.0162)
Prandtl number → Prandtlova značajka
Prandtl number (Pr) is a dimensionless quantity used in fluid mechanics, defined by
where η is viscosity, ρ is density, and a is thermal diffusivity.
Citing this page:
Generalic, Eni. "Ledište." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table