isothermal process → izotermni proces
Isothermal process is a thermodynamic process in which the temperature of the system does not change.
isotropic crystal → izotropni kristal
The measured properties of an isotropic crystal are independent of the axis of testing. Opposite of anisotropic.
cryogenic fractionation → kriogena frakcinacija
Cryogenic fractionation is a process of separation of gases by cooling them until they enter their liquid state. Large scale gas production companies use this method to produce liquid oxygen, liquid nitrogen etc. Gases have different boiling points (the temperature at which they change from liquid to gas). Oxygen has a boiling point of -183 °C, and nitrogen a boiling point of -195.8 °C. Therefore by cooling the gas mixture to -183 °C, the oxygen can be collected as liquid and the nitrogen remains its gaseous form.
cyclic compound → ciklički spoj
Cyclic describing a compound that has a ring of atoms in its molecules. In homocyclic compounds all the atoms in the ring are of the same type, e.g. benzene (C6H6) and cyclohexane (C6H12). These two examples are also examples of carbocyclic compounds; i.e. the rings are made of carbon atoms. If different atoms occur in the ring, as in pyridine (C5H5N), the compound is said to be heterocyclic.
cyclic voltammetry → ciklička voltametrija
Cyclic voltammetry (CV) is an electrochemical measuring technique used for the determination of the kinetics and mechanism of electrode reactions. The potential of the working electrode is controlled (typically with a potentiostat) and the current flowing through the electrode is measured. It is a linear-weep voltammetry with the scan continued in the reverse direction at the end of the first scan. This cycle can be repeated a number of times, and is used for corrosion studies.
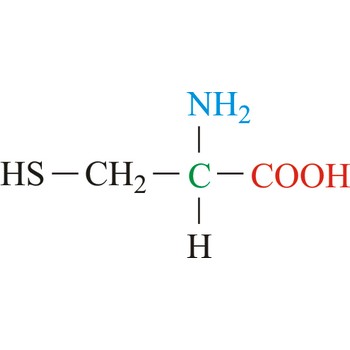
cysteine → cistein
Cysteine is neutral amino acids with polar side chains. Because of its high reactivity, the thiol group of cysteine has numerous biological functions. It serves as a potent nucleophile and metal ligand (particularly for iron and zinc), but is best known for its ability to form disulfide bonds, which often make an important contribution to the stability of extracellular proteins. Cysteine is a non-essential amino acid, which means that it is biosynthesized in humans.
- Abbreviations: Cys, C
- IUPAC name: 2-amino-3-sulfanylpropanoic acid
- Molecular formula: C3H7NO2S
- Molecular weight: 121.16 g/mol
crystal system → kristalni sustav
Crystal system is a method of classifying crystalline substances on the basis of their unit cell. There are seven unique crystal systems. The simplest and most symmetric, the cubic (or isometric) system, has the symmetry of a cube. The other six systems, in order of decreasing symmetry, are hexagonal, tetragonal, rhombohedral (also known as trigonal), orthorhombic, monoclinic and triclinic.
|
Crystal system
|
Unit-cell
|
Conditions on unit-cell edges and angles |
|
cubic |
 |
a=b=c α=β=γ=90° |
|
hexagonal |
 |
a≠c α=γ=90° β=120° |
|
tetragonal |
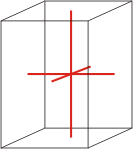 |
a=b≠c α=β=γ=90° |
|
rhombohedral |
 |
a=b=c α=β=γ≠90° |
|
orthorhombic |
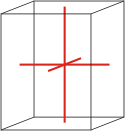 |
a≠b≠c α=β=γ=90° |
|
monoclinic |
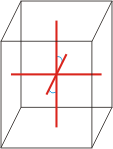 |
a≠b≠c α=γ=90°≠β |
|
triclinic |
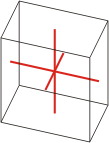 |
a≠b≠c α≠β≠γ≠90° |
Citing this page:
Generalic, Eni. "Ledište." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table