gas thermometer → plinski termometar
Gas thermometer is a device for measuring temperature in which the working fluid is a gas.
glass transition temperature → temperatura staklastog prijelaza
Glass transition temperature (Tg) is the temperature at which an amorphous polymer is transformed, in a reversible way, from a viscous or rubbery condition to a hard and relatively brittle one.
clay triangle → trokut za žarenje
Clay triangle is a piece of laboratory equipment used in the process of heating substances by a Bunsen burner (e.g. to support a crucible when it’s being heated).
collision theory → teorija sudara
Collision theory is theory that explains how chemical reactions take place and why rates of reaction alter. For a reaction to occur the reactant particles must collide. Only a certain fraction of the total collisions cause chemical change; these are called successful collisions. The successful collisions have sufficient energy (activation energy) at the moment of impact to break the existing bonds and form new bonds, resulting in the products of the reaction. Increasing the concentration of the reactants and raising the temperature bring about more collisions and therefore more successful collisions, increasing the rate of reaction.
heat transfer → prijenos topline
From observations and experiments it has been found that heat energy can be transferred from one position to another through three different modes: conduction, convection and radiation.
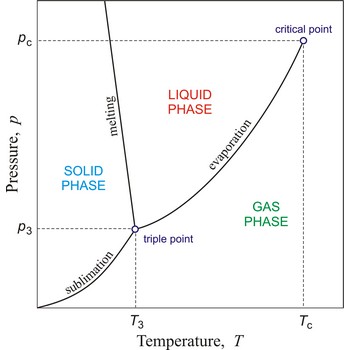
critical temperature → kritična temperatura
Critical temperature is the temperature of the liquid-vapour critical point, that is, the temperature above which a gas cannot be liquefied by an increase of pressure.
Curie temperature → Curiejeva temperatura
For a ferromagnetic material, Curie temperature or Curie point (TC) is the critical temperature above which the material becomes paramagnetic. For iron the Curie point is 760 °C and for nickel 356 °C. It was named after the French physicist Pierre Curie (1859-1906).
kinetic theory → kinetička teorija
Kinetic theory explains the behaviour of solids, liquids and gases and their state changes dependable upon motion of particles they are made of.
Dalton’s atomic theory → Daltonova atomska teorija
Dalton’s atomic theory is a theory of chemical combination, first stated by John Dalton in 1803. It involves the following postulates:
1. Elements consist of indivisible small particles (atoms).
2. All atoms of the same element are identical; different elements have different types of atom.
3. Atoms can neither be created nor destroyed.
4. ’Compound elements’ (i.e. compounds) are formed when atoms of different elements join in simple ratios to form ’compound atoms’ (i.e. molecules).
Dalton also proposed symbols for atoms of different elements (later replaced by the present notation using letters).
Citing this page:
Generalic, Eni. "Ledište." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table