Tyndall’s effect → Tyndallov efekt
Tyndall’s effect occurs when light disperses on colloid particles. This phenomenon can be seen when a ray of light enters in dark room through a small hole. In the beam some dust particles of colloid dimensions can be seen sparkling.
ultracentrifuge → ultracentrifuga
Ultracentrifuge is a high-speed centrifuge used in the separation of colloidal or submicroscopic particles. The ultracentrifuge can generate forces thousands or millions of times stronger than the force of gravity.
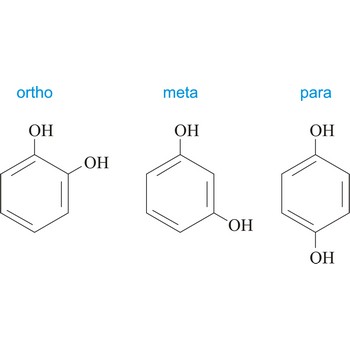
meta position → meta položaj
Meta position in organic chemistry is the one in which there are two same functional groups tied to a ring of benzene in position 1 and 3. The abbreviation m- is used, for example, m-Hydroquinone is 1,3-dihydroxybenzene.
metal → metal
Metals are materials in which the highest occupied energy band (conduction band) is only partially filled with electrons.
Their physical properties generally include:
- They are good conductors of heat and electricity. The electrical conductivity of metals generally decreases with temperature.
- They are malleable and ductile in their solid state.
- They show metallic lustre.
- They are opaque.
- They have high density.
- They are solids (except mercury)
- They have a crystal structure in which each atom is surrounded by eight to twelve near neighbours
Their chemical properties generally are:
- They have one to four valence electrons.
- They have low ionisation potentials; they readily lose electrons.
- They are good reducing agents.
- They have hydroxides which are bases or amphoteric.
- They are electropositive.
Metallic characteristics of the elements decrease and non-metallic characteristics increase with the increase of valence electrons. Also metallic characteristics increase with the number of electron shells. Therefore, there is no sharp dividing line between the metals and non-metals.
Of the 114 elements now known, only 17 show primarily non-metallic characteristics, 7 others are metalloids, and 89 may be classed as metals.
mole → mol
Mole (mol) is the SI base unit of amount of substance.
The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kg of carbon 12.
When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles. In this definition, it is understood that the carbon 12 atoms are unbound, at rest and in their ground state.
nucleotide → nukleotid
Nucleotides are the components that made up nucleic acids. They have three major components: the first component is a nitrogenous base, which is derivative of one of two parent compounds, pyrimidine or purine; the second is a pentose, or five carbon sugar group; the third is a unit of phosphate. Each group of three nucleotides in a gene is known as a codon. Whenever the phosphate group is not present, a nucleotide becomes a nucleoside.
weak base → slaba baza
Weak base is a base that only partially dissociates into ions in solution. Weak bases are weak electrolytes. Ammonia is an example of a weak base
weak electrolyte → slabi elektrolit
Weak electrolytes are those electrolytes which in water solutions dissociate only partially, giving ions and which are in equilibrium with undissociated molecules. Their water solutions conduct electric current weakly. For example, acetic acid partially dissociates into acetate ions and hydrogen ions, so that an acetic acid solution contains both molecules and ions.
ortho position → ortho položaj
Ortho position in organic chemistry is the one in which there are two same functional groups, tied to a ring of benzene in the positions 1 and 2. The abbreviation o- is used, for example, o-Hydroquinone is 1,2-dihydroxybenzene.
photochemical reaction → fotokemijska reakcija
Photochemical reactions are those reactions which are conducted under the influence of light that is under the influence of ultraviolet, visible and infrared part of the light spectrum. Some systems can be influenced only by radiation that is absorbed by that system. Photochemical reactions are for example photosynthesis, creation of photography, generation of phosgene, creation of hydrochloride etc.
Citing this page:
Generalic, Eni. "Leche mfgm para adultos." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table