purification → pročišćavanje
Purification is the physical or chemical process of removing contaminants from a compound. The physical processes may include sublimation, distillation, filtration, crystallisation, or extraction. The chemical processes may involve formation of a derivative, purification of the derivative and recovery of the original material in a pure form of the derivative.
racemisation → racemizacija
Racemisation is a conversion, by heat or by chemical reaction, of an optically active compound into an optically inactive form which half of the optically active substance becomes its miror image (enantiomer).
glucose → glukoza
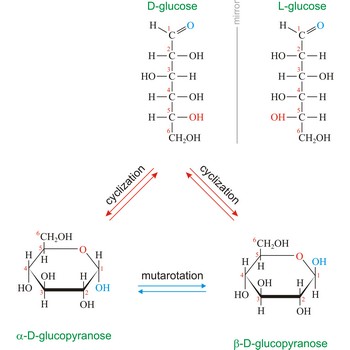
Glucose (grape sugar, blood sugar), C6H12O6, is an aldohexose (a monosaccharide sugar having six carbon atoms and an aldehyde group). An older common name for glucose is dextrose, after its dextrorotatory property of rotating plane polarized light to the right. Glucose in free (in sweet fruits and honey) or combined form (sucrose, starch, cellulose, glycogen) is is probably the most abundant organic compound in nature. During the photosynthesis process, plants use energy from the sun, water from the soil and carbon dioxide gas from the air to make glucose. In cellular respiration, glucose is ultimately broken down to yield carbon dioxide and water, and the energy from this process is stored as ATP molecules (36 molecules of ATP across all processes).
Naturally occurring glucose is D isomers (OH group on the stereogenic carbon farthest from the aldehyde group, C-5, is to the right in the Fischer projection). Although often displayed as an open chain structure, glucose and most common sugars exist as ring structures. In the α form, the hydroxyl group attached to C-1 and the CH2OH attached to C-5 are located on opposite sides of the ring. β-glucose has these two groups on the same side of the ring. The full names for these two anomers of glucose are α-D-glucopyranose and β-D-glucopyranose.
halocarbon → halogenirani ugljikovodik
Halocarbon is a compound containing no elements other than carbon, one or more halogens, and sometimes hydrogen. The simplest are compounds such as tetrachloromethane (CCl4), tetrabromomethane (CBr4), etc. The lower members of the various homologous series are used as refrigerants, propellant gases, fireextinguishing agents, and blowing agents for urethane foams. When polymerized, they yield plastics characterized by extreme chemical resistance, high electrical resistivity, and good heat resistance.
reaction mechanism → reakcijski mehanizam
Reaction mechanism is a list of all elementary reactions that occur in the course of an overall chemical reaction.
reactivity → reaktivnost
Reactivity is the tendency of a compound to chemically react with other substances or itself, resulting in the release of energy.
reversible cell → povrativi članak
Reversible cell is an electrical cell the chemical action in which can be reversed by passing through it a current opposite in direction to that generated by the cell.
Citing this page:
Generalic, Eni. "Kvantna kemija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table