non-water solution → nevodena otopina
Non-water solution is a solution in which the solvent is not a water (usualy non-polar).
diatomaceous earth → dijatomejska zemlja
Diatomaceous earth is a naturally occurring siliceous sedimentary mineral compound from microscopic skeletal remains (frustules) of diatoms, unicellular aquatic plants of microscopic size. Their fossilized remains are called diatomite and contains approximately 3000 diatom frustules per cubic millimetre.
Diatomite is relatively inert and has a high absorptive capacity, large surface area, and low bulk density. It consists of approximately 90 % silica, and the remainder consists of compounds such as aluminum and iron oxides. The fine pores in the diatom frustules make diatomite an excellent filtering material for waters, beverages, oils, chemicals, as well as many other products.
dielectric constant → dielektrična konstanta
Dielectric constant or permittivity (ε) is an index of the ability of a substance to attenuate the transmission of an electrostatic force from one charged body to another. The lower the value, the greater the attenuation. The standard measurement apparatus utilises a vacuum whose dielectric constant is 1. In reference to this, various materials interposed between the charged terminal have the following value at 20 °C:
| vacuum | 1 |
| air | 1.00058 |
| glass | 3 |
| benzene | 2.3 |
| acetic acid | 6.2 |
| ammonia | 15.5 |
| ethanol | 25 |
| glycerol | 56 |
| water | 81 |
The exceptionally high value for water accounts for its unique behaviour as a solvent and in electrolytic solutions. Dielectric constant values decrease as the temperature rises.
dioxin → dioksin
Dioxin is a general term that describes a group of hundreds of chemicals that are highly persistent in the environment. The most toxic compound is 2,3,7,8-tetrachlorodibenzo-p-dioxin or TCDD. The toxicity of other dioxins and chemicals like PCBs that act like dioxin are measured in relation to TCDD. Dioxin is formed as an unintentional by-product of many industrial processes involving chlorine such as waste incineration, chemical and pesticide manufacturing and pulp and paper bleaching. Dioxin was the primary toxic component of Agent Orange, found at Love Canal in Niagara Falls, NY and was the basis for evacuations at Times Beach, MO and Seveso, Italy.
Dioxin is formed by burning chlorine-based chemical compounds with hydrocarbons. The major source of dioxin in the environment comes from waste-burning incinerators of various sorts and also from backyard burn-barrels. Dioxin pollution is also affiliated with paper mills which use chlorine bleaching in their process, with the production of Polyvinyl Chloride (PVC) plastics, and with the production of certain chlorinated chemicals (like many pesticides).
ohm → om
Ohm (Ω) is the SI derived unit of electric resistance. The ohm is the electric resistance between two points of a conductor when a constant difference of potential of one volt, applied between these two points, produces in this conductor a current of one ampere, this conductor not being the source of electromotive force (Ω = V/A). The unit was named after the German physicist Georg Simon Ohm (1789-1854).
dipole → dipol
Dipole is a pair of separated opposite electric charges. Electric dipole is an assemblage of atoms or subatomic particles having equal electric charges of opposite sign separated by a finite distance. In the case of HCl, the electrons are attracted towards the more electronegative chlorine atom.
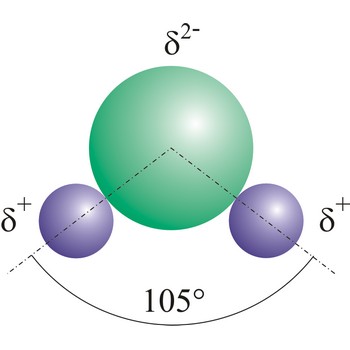
dipole molecule → dipolna molekula
Dipole molecules are created when mutual electronic pair at covalent bond is asymmetrical. If different atoms are bonded by a covalent bond, which can have different electron affinity, then the the atom with greater electron affinity will attract the electron pairs more strongly. In this way an asymmetrical distribution of negative charge appears in a molecule, so one part of the molecule becomes relatively negatively (the one closer to the electron pair) and the other becomes relatively positively charged.
dissociation → disocijacija
Dissociation is the process by which a chemical combination breaks up into simpler constituents as a result of either added energy (dissociated by heat), or the effect of a solvent on a dissolved polar compound (electrolytic dissociation). It may occur in the gaseous, solid, or liquid state, or in a solution.
An example of dissociation is the reversible reaction of hydrogen iodide at high temperatures
The term dissociation is also applied to ionisation reactions of acids and bases in water. For example
which is often regarded as a straightforward dissociation into ions
Citing this page:
Generalic, Eni. "Kristalna voda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table