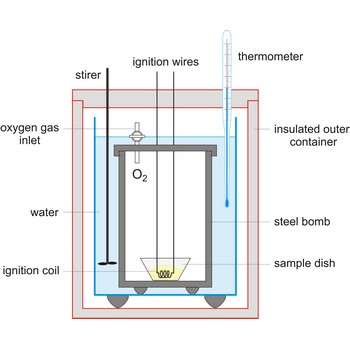
bomb calorimeter → kalorimetrijska bomba
Bomb calorimeter is a type of constant-volume calorimeter used in measuring the heat of combustion of samples which can be burned in oxygen. Four essential parts are required in any bomb calorimeter:
- a bomb or vessel in which the combustible charges can be burned,
- a bucket or container for holding the bomb in a measured quantity of water, together with a stirring mechanism,
- an insulating jacket to protect the bucket from transient thermal stresses during the combustion process, and
- a thermometer or other sensor for measuring temperature changes within the bucket.
borane → borani
Borane is any of the group of compounds of boron and hydrogen (B2H6, B4H10, B5H9, B5H11...), many of which can be prepared by action of acid on magnesium boride (Mg3B2). Boranes are a remarkable group of compounds in that their structures cannot be described using the conventional two-electron covalent bond model.
equivalent weight → ekvivalentna masa
Equivalent weight of a substance participating in a neutralization reaction is that mass of substance (molecule, ion, or paired ion) that either reacts with or supplies 1 mol of hydrogen ions in that reaction.
Equivalent weight of a substance participating in an oxidation/reduction reaction is that weight which directly or indirectly produces or consumes 1 mol of electrons.
Born-Haber cycle → Born-Haberov kružni proces
Born-Haber cycle is a cycle of reactions used for calculating the lattice energies of ionic crystalline solids. For a compound MX, the lattice energy is the enthalpy of the reaction
The standard enthalpy of formation of the ionic solid is the enthalpy of the reaction
The cycle involves equating this enthalpy (which can be measured) to the sum of the enthalpies of a number of steps proceeding from the elements to the ionic solid. The steps are:
1) Atomization of the metal
2) Atomization of the nonmetal
3) Ionisation of the metal
This is obtained from the ionisation potential.
4) Ionisation of the nonmetal
This is electron affinity.
5) Formation of the ionic solids
Equation of the enthalpies gives
from which ΔHL can be found.
Bragg angle → Braggov kut
Bragg angle (Θ) is the angle between an incident X-ray beam and a set of crystal planes for which the secondary radiation displays maximum intensity as a result of constructive interference. British physicist Sir William Henry Bragg and his son Sir William Lawrence Bragg developed a simple relation for scattering angles, now call Bragg’s law.
which relates the angle θ between a crystal plane and the diffracted X-ray beam, the wavelength λ of the x-rays, the crystal plane spacing d, and the diffraction order n (any integer).
The diffraction experiment as presently considered is intended to provide quantitative information on the lattice constant and shape characteristics of the unit cell.
Fermi level → Fermijev nivo
Fermi level is the highest energy of occupied states in a solid at zero temperature. The Fermi level in conductors lies in the conduction band, in insulators it lies in the valence band, and in semiconductors it falls in the gap between the conduction band and the valence band. It was named after the Italian physicst Enrico Fermi (1901 - 1954).
fire-damp → plin praskavac
Fire-damp is a mixture of two volume parts of hydrogen and one volume part of oxygen which, if set on fire, strongly explodes, the flame giving of a very high temperature (2 000 °C).
brass → mjed
Brasses are alloys of copper and zinc (generally 5 % to 40 %). Brass has been known to man since prehistoric times, long before zinc itself was discovered. It was produced by melting copper together with calamine, a zinc ore. Its ductility reaches a maximum with about 30 % zinc and its tensile strength with 45 % although this property varies greatly with the mechanical and heat treatment of the alloy. Typical applications included gears, plumbing ware fittings, adapters, valves and screw machine products. The French horn is a valved brass wind instrument.
Brass may contain small amounts of other alloying elements, such as aluminum, lead, tin, or nickel. Lead can be added as an alloying element resulting in a brass that can be rapidly machined and produces minimal tool wear. Additions of aluminium, iron and manganese to brass improve strength, whilst silicon additions improve wear resistance. Brass containing tin (< 2 % ) is less liable to corrosion in seawater; it is sometimes called naval brass and is used in naval construction.

bromine → brom
Bromine was discovered by Antoine J. Balard (France) in 1826. The origin of the name comes from the Greek word bromos meaning stench. It is reddish-brown liquid with suffocating, irritating fumes. Gives off poisonous vapour. Causes severe burns. Oxidizer. Bromine occurs in compounds in sea water. It was once used in large quantities to make a compound that removed lead compound build up in engines burning leaded gasoline. Now it is primarily used in dyes, disinfectants and photographic chemicals.
Citing this page:
Generalic, Eni. "Kristalna voda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table