body-centered cubic lattice → prostorno centrirana kubična rešetka
Body-centered cubic lattice (bcc or cubic-I), like all lattices, has lattice points at the eight corners of the unit cell plus an additional points at the center of the cell. It has unit cell vectors a = b = c and interaxial angles α=β=γ=90°.
The simplest crystal structures are those in which there is only a single atom at each lattice point. In the bcc structures the spheres fill 68 % of the volume. The number of atoms in a unit cell is two (8 × 1/8 + 1 = 2). There are 23 metals that have the bcc lattice.
body-centered orthorhombic lattice → prostorno centrirana ortorompska rešetka
Body-centered orthorhombic lattice (orthorhombic-I), like all lattices, has lattice points at the eight corners of the unit cell plus an additional points at the center of the cell. It has unit cell vectors a≠b≠c and interaxial angles α=β=γ=90°.
dehydrogenation → dehidrogenacija
Dehydrogenation is a chemical reaction in which hydrogen is removed from a compound. Dehydrogenation of organic compounds converts single carbon-carbon bonds into double bonds. It is usually affected by means of a metal catalyst or in biological systems by enzyme dehydrogenases.
deuterium lamp → deuterijeva svjetiljka
Deuterium or hydrogen lamp is used as a source of continuous radiation in UV part of the spectrum.
dilution → razrjeđivanje
Dilution is the action of diluting or reducing the strength or concentration of a liquid, usually by the addition of water.
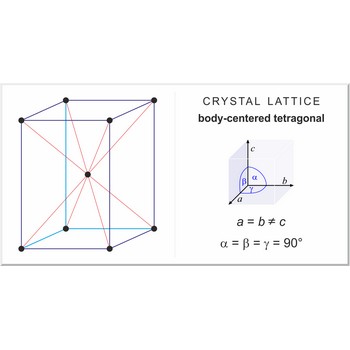
body-centered tetragonal lattice → prostorno centrirana tetragonska rešetka
Body-centered tetragonal lattice (tetragonal-I), like all lattices, has lattice points at the eight corners of the unit cell plus an additional points at the center of the cell. It has unit cell vectors a=b≠c and interaxial angles α=β=γ=90°.
Citing this page:
Generalic, Eni. "Kristalna voda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table