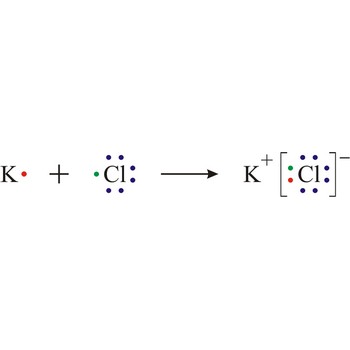hydrogen bond → vodikova veza
Hydrogen is a bond formed by a hydrogen atom to an electronegative atom, and is denoted by dashed lines H-X---H-B. A hydrogen atom covalently bound to an oxygen (electronegative atom) has a significant positive charge and can form a weak bond to another electronegative atom.
ionic bond → ionska veza
Ionic bond is a strong force of attraction holding atoms together in a molecule or crystal. Typically chemical bonds have energies of about 100 kJ mol-1. Ionic bond is a bond at which one of the participants, during the procedure of bonding, gives away its unpaired electrons to another atom so that both can achieve electron arrangement of the closest noble gas. In order to form an ionic bond one of the atoms must cross to the positively charged ion by losing certain number of electrons and the other atom must receive those electrons and cross to the negatively charged ion.
peptide bond → peptidna veza
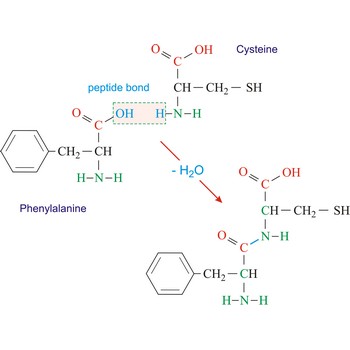
Peptide bond emerges when two amino acid join in a way that the carbon atom from one connects with the nitrogen atom from the other (creating a C-N bond).
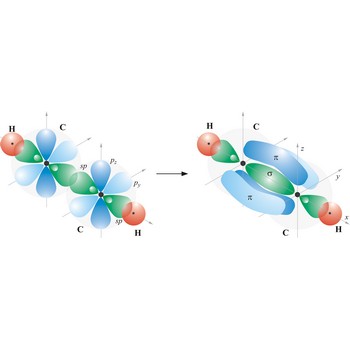
sigma bond → sigma veza
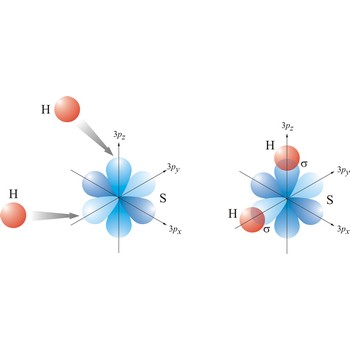
Most single bonds are sigma bonds (σ-bond). In the valence bond theory, a sigma bond is a valence bond that is symmetrical around the imaginary line between the bonded atoms.
triple bond → trostruka veza
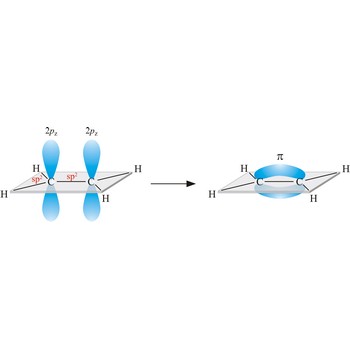
Triple bond. (≡) is a covalent bond that involves 3 bonding pairs. In the valence bond theory, one of the bonds in a triple bond is a sigma bond and the other two are pi bonds. For example, the central bond in acetylene is a triple bond: H-C≡C-H.
conjugated protein → konjugirani protein
Conjugated proteins are proteins which have a prostetic group as a part of their structure which is bonded with one or more amino acids of the same protein.
valence bond theory → teorija valentne veze
Valence bond theory is a theory that explains the shapes of molecules in terms of overlaps between half-filled atomic orbitals, or half filled hybridised orbitals.
Citing this page:
Generalic, Eni. "Konjugirana veza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table