condensation → kondenzacija
1. Condensation is a process of changing from a gaseous to a liquid or solid state, usually done by cooling.
2. Condensation, in colloid systems, is a process where smaller particle join in one colloid size particle
3. Condensation, in chemical terms, is a sort of chemical reaction in which small molecules like water, carbon dioxide, or ammonia single out.
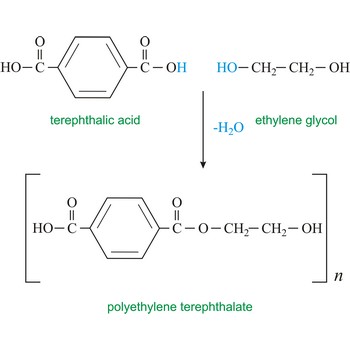
condensational polymerisation → kondenzacijska polimerizacija
Condensational polymerisation is a reaction of polymerisation in which monomers together create a polymer by losing small molecules like water.
colloid → koloid
Colloids are systems in which there are two or more phases, with one (the dispersed phase) distributed in the other (the continuous phase). Moreover, at least one of the phases has small dimensions, in the range between 1 nm and 1 μm (10-9 m – 10-6 m). Dimension, rather than the nature of the material, is characteristic. In this size range, the surface area of the particle is large with respect to its volume so that unusual phenomena occur, e.g., the particles do not settle out of the suspension by gravity and are small enough to pass through filter membranes. Macromolecules (proteins and other high polymers) are at the lower limit of this range; the upper limit is usually taken to be the point at which the particles can be resolved in an optical microscope.
Colloidal particles may be gaseous, liquid, or solid, and occur in various types of suspensions:
Sols - dispersions of small solid particles in a liquid.
Emulsions - colloidal systems in which the dispersed and continuous phases are both liquids.
Gels - colloids in which both dispersed and continuous phases have a three-dimensional network throughout the material.
Aerosols - colloidal dispersions of liquid or solid particles in a gas.
Foams - dispersions of gases in liquids or solids.
copolymer → kopolimer
Copolymers are also known as heteropolymers. They are made from two (or more) different monomers, which usually undergo a condensation reaction with the elimination of a simple molecule, such as ammonia or water. A typical example is the condensation of 1,6-diaminohexane (hexamethylenediamine) with hexanedioic acid (adipic acid) to form nylon 6,6.
The properties of a polymeric plastic can most easily be modified if it is a copolymer of two or more different monomers, e.g. acrylonitrile-butadiene-styrene copolymer (ABS). Varying the proportions of the component monomers can preselect its properties.
polycondensational polymer → polikondenzacijski polimer
Polycondensational polymers are compounds which are obtained by condensation polymerization with successive repetitions of the condensation reaction.
distilled water → destilirana voda
Distilled water is water purified by distillation so as to free it from dissolved salts and other compounds. Distilled water in equilibrium with the carbon dioxide in the air has conductivity of about 0.8×10-6 S cm-1. Repeated distillation in vacuum can bring conductivity down to 0.043×10-6 S cm-1 at 18 °C. The limiting conductivity is due to self ionisation
Wagner tube → Wagnerova cijev
Wagner tube or drop catcher impedes the movement of distillate drops from the distillation flask to the condensing chamber.
Citing this page:
Generalic, Eni. "Kondenzacija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table