oxygen → kisik
Oxygen was discovered by Joseph Priestley (England) in 1774. The origin of the name comes from the Greek words oxy genes meaning acid and forming (acid former). It is colourless, odourless gas; pale blue liquid. Extremely reactive. Forms oxides with nearly all other elements except noble gases. It is the most abundant element in the earth’s crust and makes up almost 21 % of the atmosphere. Oxygen is obtained primarily from liquid air by fractional distillation. Small amounts are made in the laboratory by electrolysis of water. Used in steel making, welding and supporting life. Naturally occurring ozone (O3) in the upper atmosphere shields the earth from ultraviolet radiation.
abundance of elements → rasprostranjenost elemenata
Elements in nature are mostly found in different compounds and, rarely, in the free (elementary) state. In Earth’s crust the most abundant of all elements is oxygen (with 49.5 %), then silicon (25 %), aluminium (7.5 %), iron (4.7 %), calcium (3.4 %), sodium (2.6 %), potassium (2.4 %), magnesium (1.9 %) and hydrogen (1.9 %). These nine elements make up almost 99 % of the Earth’s composition.
activated charcoal → aktivni ugljen
Activated charcoal or activated carbon is charcoal that has been activated for adsorption by steaming or by heating in a vacuum. Charcoal is obtained by burning wood, nutshells, coconut husks or other materials. Charcoal becomes activated by heating it with steam to approximately 1000 °C in the absence of oxygen.
The chemical nature of amorphous carbon, combined with a high surface area makes it an ideal medium for the adsorption of organic chemicals. A single gram of such material can have 400 m2 to 1 200 m2 square meters of surface area. Activated charcoal is widely used to decolorize liquids, recover solvents, and remove toxins from water and air.
autoxidation → autooksidacija
Autoxidation, autooxidation is a oxidation is caused by exposure to air. Rust is an example of autoxidation. Autoxidation makes ether taken from half-filled bottles very dangerous, because air oxidises ether to highly explosive organic peroxides.
basic Bessemer process → bazni Bessemerov proces
Basic Bessemer process is the process of steelmaking in a way that steel, melted iron and limestone are put in the blast-furnace after which the metal oxygen is released in gushes in order to oxidise the impurities.
berkelium → berkelij
Berkelium was discovered by Stanley G. Thompson, Albert Ghiorso and Glenn T. Seaborg (USA) in 1949. Named after Berkeley, a city in California, home of the University of California, USA. It is synthetic radioactive metal. Berkelium was made by bombarding americium with alpha particles.
allotrope → alotrop
Allotropes are the elements which exist in two or more different forms in the same physical state. Allotropes generally differ in physical properties and may also differ in chemical activity.
Diamond, graphite and fullerenes are three allotropes of the element carbon. Graphite is a soft, black, slippery substance; by contrast, diamond is one of the hardest substances known. The different properties of the allotropes arise from their chemical structures. Diamonds typically crystallize in the cubic crystal system and consist of tetrahedrally bonded carbon atoms. Graphite crystallizes in the hexagonal system. In the fullerenes, the carbon atoms taking the form of a hollow sphere, ellipsoid, or tube.
In some cases, the allotropes are stable over a temperature range, with a definite transition point at which one changes into the other. For instance, tin has two allotropes: white (metallic) tin stable above 13.2 °C and grey (nonmetallic) tin stable below 13.2 °C.
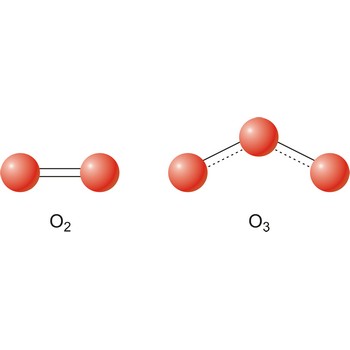
The term allotropes may also be used to refer to the molecular forms of an element. Ozone is a chemically active triatomic allotrope of the element oxygen.
allotropic modification → alotropska modifikacija
Different substances of the same elementary system are called allotropes or allotropic modifications. In the case of oxygen, there are two allotropic modifications: "normal" dioxygen (O2) and trioxygen (O3) or ozone.
Citing this page:
Generalic, Eni. "Kisik." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table