active site → aktivno mjesto
Active site is a pocket or crevice on an enzyme molecule that fits reactant molecules like a hand in a glove. The active site lowers the activation energy for reaction
adenosine triphosphate → adenozin trifosfat
Adenosine triphosphate (ATP) is nucleotide that is of fundamental importance as a carrier of chemical energy in all living organisms. It consists of adenin linked to D-ribose).
Arrhenius equation → Arheniusova jednadžba
In 1889, Svante Arrhenius explained the variation of rate constants with temperature for several elementary reactions using the relationship
where the rate constant k is the total frequency of collisions between reaction molecules A times the fraction of collisions exp(-Ea/RT) that have an energy that exceeds a threshold activation energy Ea at a temperature of T (in kelvin). R is the universal gas constant.
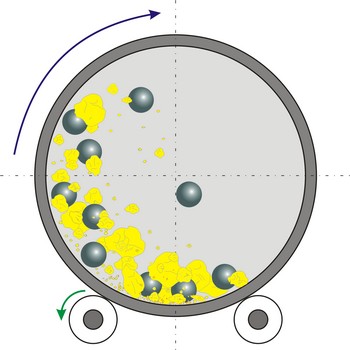
ball mill → kuglični mlin
Ball mill is a grinder for reducing hard materials to powder. The grinding is carried out by the pounding and rolling of a charge of steel or ceramic balls carried within the cylinder. The cylinder rotates at a relatively slow speed, allowing the balls to cascade through the mill base, thus grinding or dispersing the materials.
Type of ball mills, centrifugal and planetary mills, are devices used to rapidly grind materials to colloidal fineness (approximately 1 μm and below) by developing high grinding energy via centrifugal and/or planetary action.
conduction → kondukcija
This process occurs most significantly in solids. The atoms or molecules in a solid state do not leave their mean positions, but continue to vibrate about their mean positions. They transfer heat energy from one atom to another. This happens because of the coupling between them due to mutually attractive forces.
cosmic rays → kozmičke zrake
Cosmic rays are high energy (1015 eV- 1017 eV) nuclear particles, electrons, and photons, originating mostly outside the solar system, which continually bombard the Earth’s atmosphere.
degenerate orbitals → degenerirane orbitale
Degenerate orbitals are orbitals with the same energy. This degeneracy can sometimes be "lifted" by external electric or magnetic fields.
electron spin → elektronski spin
Electron spin (s) is the quantum number, equal to 1/2, that specifies the intrinsic angular momentum of the electron.
battery → baterija
Battery a device that converts chemical energy to electrical energy. The process underlying the operation of a battery involves a chemical reaction in which electrons are transferred from one chemical species to another. This process is carried out in two half-reactions, one that involves the loss of electrons and one that involves their gain. The battery is an electrochemical cell divided in two half-cells, and reaction proceeds when these are connected together by an electrically conducting pathway. The passage of electrons from one half-cell to the other corresponds to an electric current. Each half-cell contains an electrode in contact with the reacting species. The electrode which passes electrons into the circuit when battery discharges is called anode and is negative terminal. The electrode which receives electrons is called cathode, and is the battery’s positive terminal. The electrical circuit is completed by an electrolyte, an electrically conducting substance placed between the two electrodes which carriers a flow of charge between them. In wet cells, the electrolyte is a liquid containing dissolved ions, whose motion generates an electrical current; in dry cells the electrolyte is basely solid, for example, a solid with mobile ions or porous solid saturated with an ionic solution.
beta particle → beta-čestica
Beta particle is a charged particle emitted from a radioactive atomic nucleus either natural or manufactured. The energies of beta particles range from 0 MeV to 4 MeV. They carry a single charge; if this is negative, the particle is identical with an electron; if positive, it is a positron.
An unstable atomic nucleus changes into a nucleus of the same mass number but different proton number with the emission of an electron and an antineutrino (or a positron and a neutrino)
Citing this page:
Generalic, Eni. "Kinetička energija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table