chemical reaction equation → jednadžba kemijske reakcije
Chemical reactions are symbolically shown with chemical equations. On the left side of the equation we write formulas or substance symbols which enter the chemical reaction, reactants. On the right side formulas or substance symbols which emerge from the chemical reaction, products, are writen.
Each chemical reaction leads to an equilibrium which is moved more or less to one side (left or right). Because of that, in reversible reactions instead of = sign two opposite arrows are put
In order to write down certain chemical reaction equation all reactants and all products and their stechiometric proportions must be known. (See Chemical reaction balancing)
heat of reaction → toplina kemijske reakcije
Heat of reaction or enthalpy of reaction is the heat evolved or absorbed as a result of the complete chemical reaction of molar amounts of the reactants.
law of chemical equilibrium → zakon o kemijskoj ravnoteži
Law of chemical equilibrium (also called the law of mass action) states that the rate at which a substance reacts is proportional to its active mass (i.e. to its molar concentration). Thus, the velocity of a chemical reaction is proportional to the product of the concentration of the reactants.
accelerator → akcelerator
Accelerator is a device (machine) used for acceleration of charged particles (protons, deuterons, α-particles). Particles are accelerated under the influence of an electric field and with the help of a magnetic field are kept inside a certain space. When the particles reach enough acceleration (that is sufficient energy), they are directed on a target we wish to bomb. Best known types cyclotron, synchrotron, betatron.
Accelerator is a substance that increases the rate of chemical reaction, i.e. a catalyst.
accumulator → akumulator
Accumulator (secondary cell, storage battery) is a type of voltaic cell or battery that can be recharged by passing current through it from an external D.C. supply. The charging current reverses the chemical reactions in the cell. The common types are the lead-acid accumulator and the nickel-cadmium cell.
activated charcoal → aktivni ugljen
Activated charcoal or activated carbon is charcoal that has been activated for adsorption by steaming or by heating in a vacuum. Charcoal is obtained by burning wood, nutshells, coconut husks or other materials. Charcoal becomes activated by heating it with steam to approximately 1000 °C in the absence of oxygen.
The chemical nature of amorphous carbon, combined with a high surface area makes it an ideal medium for the adsorption of organic chemicals. A single gram of such material can have 400 m2 to 1 200 m2 square meters of surface area. Activated charcoal is widely used to decolorize liquids, recover solvents, and remove toxins from water and air.
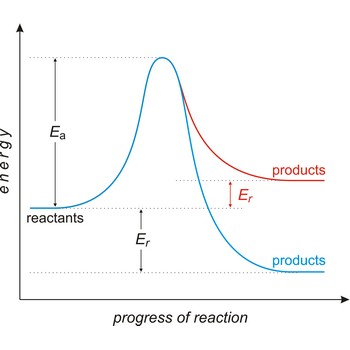
activation energy → energija aktivacije
Activation energy (Ea) is the energy that must be added to a system in order for a process to occur, even though the process may already be thermodynamically possible. In chemical kinetics, the activation energy is the height of the potential barrier separating the products and reactants. It determines the temperature dependence on the reaction rate.
allomerism → alomerija
Allomerism is the appearance of substances with different chemical composition but the same crystalline form.
Citing this page:
Generalic, Eni. "Kemijsko inženjerstvo." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table