electronegativity → elektronegativnost
Electronegativity is a parameter originally introduced by L. Pauling which describes, on a relative basis, the power of an atom to attract electrons. For example, in hydrogen chloride, the chlorine atom is more electronegative than the hydrogen and the molecule is polar, with a negative charge on the chlorine atom.
There are various ways of assigning values for the electronegativity of an element. Pauling electronegativities are based on bond dissociation energies using a scale in which fluorine, the most electronegative element, has the value 4 and francium, the lowest electronegative element, has the value 0.7.
energy → energija
Energy (E, U) is the characteristic of a system that enables it to do work. Like work itself, it is measured in joules (J).
The internal energy of a body is the sum of the potential energy and the kinetic energy of its component atoms and molecules.
Potential energy is the energy stored in a body or system as a consequence of its position, shape, or state (this includes gravitation energy, electrical energy, nuclear energy, and chemical energy).
Kinetic energy is the energy of motion and is usually defined as the work that will be done by a body possessing the energy when it is brought to rest. For a body of mass m having a speed v, the kinetic energy is mv2/2. Kinetic energy is most clearly exhibited in gases, in which molecules have much greater freedom of motion than in liquids and solids.
In an isolated system energy can be transferred from one form to another but the total energy of the system remains constant.
isomorphism → izomorfija
Isomorphism is the existence of two or more substances that have the same crystal structure, so that they form solid solutions.
enthalpy → entalpija
Enthalpy (H) is a thermodynamic property of a system defined by
where U is the internal energy of the system, p its pressure, and V its volume. J.W. Gibbs put the concept of an ensemble forward in 1902. In a chemical reaction carried out in the atmosphere the pressure remains constant and the enthalpy of reaction (ΔH), is equal to
For an exothermic reaction ΔH is taken to be negative.
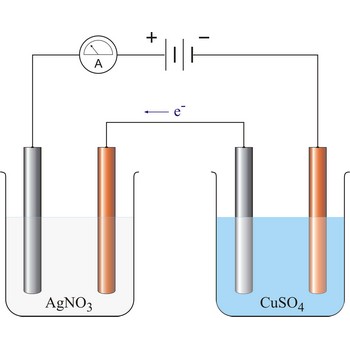
Faraday’s laws of electrolysis → Faradayevi zakoni elektrolize
Faraday’s laws of electrolysis are two laws found by British chemist and physicist Michael Faraday (1791-1867) in his experiments on electrolysis:
1. The quantity of matter extracted on the electrode is proportional to the quantity of charge (Q = I·t) which has flown in electrolysis time.
where z = number of electrons changed in reaction and F = Faraday’s constant which equals 96 487 C mol-1.
2. The masses of the elements liberated by the same quantity of electricity are directly proportional to their chemical equivalents.
96 487 C will discharge 1 mol Ag and 1/2 mol Cu. The relevant half reactions are:
law of definite composition → zakon o određenom sastavu
Law of definite composition states that the elements in a given compound are always combined in the same proportion by mass. This law form the basis for the definition of a chemical compound.
equilibrium constant → konstanta ravnoteže
The equilibrium constant (K) was originally introduced in 1863 by Norwegian chemists C.M. Guldberg and P. Waage using the law of mass action. For a reversible chemical reaction represented by the equation
chemical equilibrium occurs when the rate of the forward reaction equals the rate of the back reaction, so that the concentrations of products and reactants reach steady-state values.
The equilibrium constant is the ratio of chemical activities of the species A, B, C, and D at equilibrium.
To a certain approximation, the activities can be replaced by concentrations.
For gas reactions, partial pressures are used rather than concentrations
The units of Kp and Kc depend on the numbers of molecules appearing in the stoichiometric equation (a, b, c, and d).
The value equilibrium constant depends on the temperature. If the forward reaction is exothermic, the equilibrium constant decreases as the temperature rises. The equilibrium constant shows the position of equilibrium. A low value of K indicates that [C] and [D] are small compared to [A] and [B]; i.e. that the back reaction predominates.
The equilibrium constant is related to ΔrG°, the standard Gibbs free energy change in the reaction, by
Citing this page:
Generalic, Eni. "Kemijsko čišćenje." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table