potassium glass → kalijevo staklo
Potassium glass is a type of glass produced from potassium silicates and calcium with potassium carbonate. It dissolves harder than regular glass and it is used in production of chemical vessels.
preservative → konzervans
Preservatives are substances that will prevent the development of wood-destroying fungi, borers of various kinds, and other harmful insects that deteriorate wood.
purification → pročišćavanje
Purification is the physical or chemical process of removing contaminants from a compound. The physical processes may include sublimation, distillation, filtration, crystallisation, or extraction. The chemical processes may involve formation of a derivative, purification of the derivative and recovery of the original material in a pure form of the derivative.
glucose → glukoza
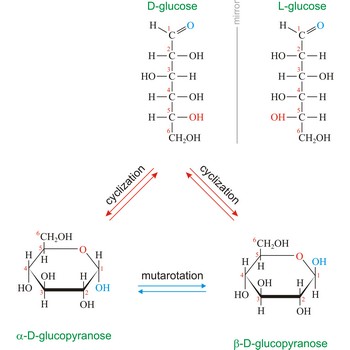
Glucose (grape sugar, blood sugar), C6H12O6, is an aldohexose (a monosaccharide sugar having six carbon atoms and an aldehyde group). An older common name for glucose is dextrose, after its dextrorotatory property of rotating plane polarized light to the right. Glucose in free (in sweet fruits and honey) or combined form (sucrose, starch, cellulose, glycogen) is is probably the most abundant organic compound in nature. During the photosynthesis process, plants use energy from the sun, water from the soil and carbon dioxide gas from the air to make glucose. In cellular respiration, glucose is ultimately broken down to yield carbon dioxide and water, and the energy from this process is stored as ATP molecules (36 molecules of ATP across all processes).
Naturally occurring glucose is D isomers (OH group on the stereogenic carbon farthest from the aldehyde group, C-5, is to the right in the Fischer projection). Although often displayed as an open chain structure, glucose and most common sugars exist as ring structures. In the α form, the hydroxyl group attached to C-1 and the CH2OH attached to C-5 are located on opposite sides of the ring. β-glucose has these two groups on the same side of the ring. The full names for these two anomers of glucose are α-D-glucopyranose and β-D-glucopyranose.
groups in periodic system of elements → skupine periodnog sustava
Periodic system of elements is divided into 18 groups of chemical elements. Elements belonging to the same group have a same number of valence electrons and similar chemical properties. Elements of main groups are in 1., 2., and in groups 13. to 18. Different groups of elements can be named according to the first element in the group (elements of boron group, elements of carbon group), or they have some special names (noble gases, halogenic elements, halyde elements, earthalkali and alkali metals).
quantum chemistry → kvantna kemija
Quantum chemistry is a theoretical branch of chemistry that concerns the application of quantum mechanics to chemical problems.
racemisation → racemizacija
Racemisation is a conversion, by heat or by chemical reaction, of an optically active compound into an optically inactive form which half of the optically active substance becomes its miror image (enantiomer).
Citing this page:
Generalic, Eni. "Kemijski simboli." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table