chemical compound formula → formula kemijskog spoja
Chemical elements are represented by their symbols, and chemical compounds are represented by a group of symbols of those elements from which the compound is composed. That group of symbols, which shows which atoms and in which number relation they are present in certain compound is called a chemical compound formula.
In a formula chemical symbols show which element is present in a certain compound, and its index shows how much of that element there is in a certain compound. From sulphuric acid formula H2SO4 we can see that one molecule of sulphuric acid consists of two atoms of hydrogen, one atom of sulphur and four atoms of oxygen.
chemical equation → kemijska jednadžba
Chemical equation is a way of denoting a chemical reaction using the symbol for the participating particles (atoms, molecules, ions, etc.); for example,
The single arrow is used for an irreversible reaction; double arrows are used for reversible reactions. When reactions involve different phases, it is usual to put the phase in brackets after the symbol.
| s | = | solid |
| l | = | liquid |
| g | = | gas |
| aq | = | aqueous |
The numbers a, b, c, and d, showing the relative numbers of molecules reacting, are called the stoichiometric coefficients. The convention is that stoichiometric coefficients are positive for reactants and negative for products. If the sum of the coefficients is zero, the equation is balanced.
chemical reaction equation → jednadžba kemijske reakcije
Chemical reactions are symbolically shown with chemical equations. On the left side of the equation we write formulas or substance symbols which enter the chemical reaction, reactants. On the right side formulas or substance symbols which emerge from the chemical reaction, products, are writen.
Each chemical reaction leads to an equilibrium which is moved more or less to one side (left or right). Because of that, in reversible reactions instead of = sign two opposite arrows are put
In order to write down certain chemical reaction equation all reactants and all products and their stechiometric proportions must be known. (See Chemical reaction balancing)
chemical weapons → kemijsko oružje
The Chemical Weapons Convention, article 2, paragraph 1 defines chemical weapons thus:
Chemical weapons means the following, together or separately:
(a) Toxic chemicals and their precursors, except where intended for purposes not prohibited under this Convention, as long as the types and quantities are consistent with such purposes;
(b) Munitions and devices, specifically designed to cause death or other harm through the toxic properties of those toxic chemicals specified in subparagraph (a), which would be released as a result of the employment of such munitions and devices;
(c) Any equipment specifically designed for use directly in connection with the employment of munitions and devices specified in subparagraph (b).
heat of reaction → toplina kemijske reakcije
Heat of reaction or enthalpy of reaction is the heat evolved or absorbed as a result of the complete chemical reaction of molar amounts of the reactants.
law of chemical equilibrium → zakon o kemijskoj ravnoteži
Law of chemical equilibrium (also called the law of mass action) states that the rate at which a substance reacts is proportional to its active mass (i.e. to its molar concentration). Thus, the velocity of a chemical reaction is proportional to the product of the concentration of the reactants.
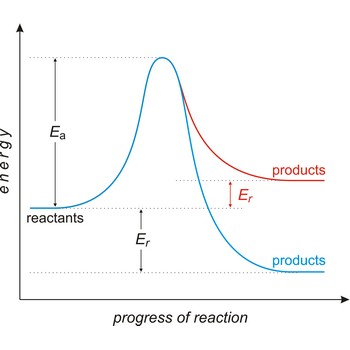
activation energy → energija aktivacije
Activation energy (Ea) is the energy that must be added to a system in order for a process to occur, even though the process may already be thermodynamically possible. In chemical kinetics, the activation energy is the height of the potential barrier separating the products and reactants. It determines the temperature dependence on the reaction rate.
electrochemical series → elektrokemijski niz
Electrochemical series is a series of chemical elements arranged in order of their standard electrode potentials. The hydrogen electrode
is taken as having zero electrode potential. An electrode potential is, by definition, a reduction potential.
Elements that have a greater tendency than hydrogen to lose electrons to their solution are taken as electropositive; those that gain electrons from their solution are below hydrogen in the series and are called electronegative.
The series shows the order in which metals replace one another from their salts; electropositive metals will replace hydrogen from acids.
energy → energija
Energy (E, U) is the characteristic of a system that enables it to do work. Like work itself, it is measured in joules (J).
The internal energy of a body is the sum of the potential energy and the kinetic energy of its component atoms and molecules.
Potential energy is the energy stored in a body or system as a consequence of its position, shape, or state (this includes gravitation energy, electrical energy, nuclear energy, and chemical energy).
Kinetic energy is the energy of motion and is usually defined as the work that will be done by a body possessing the energy when it is brought to rest. For a body of mass m having a speed v, the kinetic energy is mv2/2. Kinetic energy is most clearly exhibited in gases, in which molecules have much greater freedom of motion than in liquids and solids.
In an isolated system energy can be transferred from one form to another but the total energy of the system remains constant.
fugacity → fugacitet
Fugacity (f) is a thermodynamic function used in place of partial pressure in reactions involving real gases and mixtures. For a component of a mixture, it is defined by
where μ is the chemical potential.
The fugacity of a gas is equal to the pressure if the gas is ideal. The fugacity of a liquid or solid is the fugacity of the vapour with which it is in equilibrium. The ratio of the fugacity to the fugacity in some standard state is the activity.
Citing this page:
Generalic, Eni. "Kemijski potencijal." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table