electronegativity → elektronegativnost
Electronegativity is a parameter originally introduced by L. Pauling which describes, on a relative basis, the power of an atom to attract electrons. For example, in hydrogen chloride, the chlorine atom is more electronegative than the hydrogen and the molecule is polar, with a negative charge on the chlorine atom.
There are various ways of assigning values for the electronegativity of an element. Pauling electronegativities are based on bond dissociation energies using a scale in which fluorine, the most electronegative element, has the value 4 and francium, the lowest electronegative element, has the value 0.7.
oxidation → oksidacija
The term oxidation originally meant a reaction in which oxygen combines chemically with another substance. More generally, oxidation is a part of a chemical reaction in which a reactant loses electrons (increases oxidation number). Simultaneous reduction of a different reactant must occur (redox reaction).
permeability → permeabilnost
Permeability (Latin permeare, to pass through) is a passage or diffusion of a gas, vapour, liquid, or solid through a material without physically or chemically affecting it.
polymorphic transition → polimorfni prijelaz
Polymorphic transition is a reversible transition of a solid crystalline phase at a certain temperature and pressure to another phase of the same chemical composition with a different crystal structure. For examples, the transitions of quartz (SiO2) at 1 143 K to tridymite, and at 1 743 K to cristobalite.
enthalpy → entalpija
Enthalpy (H) is a thermodynamic property of a system defined by
where U is the internal energy of the system, p its pressure, and V its volume. J.W. Gibbs put the concept of an ensemble forward in 1902. In a chemical reaction carried out in the atmosphere the pressure remains constant and the enthalpy of reaction (ΔH), is equal to
For an exothermic reaction ΔH is taken to be negative.
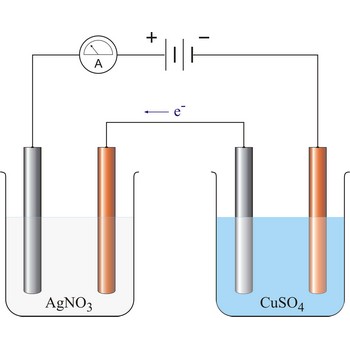
Faraday’s laws of electrolysis → Faradayevi zakoni elektrolize
Faraday’s laws of electrolysis are two laws found by British chemist and physicist Michael Faraday (1791-1867) in his experiments on electrolysis:
1. The quantity of matter extracted on the electrode is proportional to the quantity of charge (Q = I·t) which has flown in electrolysis time.
where z = number of electrons changed in reaction and F = Faraday’s constant which equals 96 487 C mol-1.
2. The masses of the elements liberated by the same quantity of electricity are directly proportional to their chemical equivalents.
96 487 C will discharge 1 mol Ag and 1/2 mol Cu. The relevant half reactions are:
positive pole → pozitivni pol
Positive pole is that half-cell in the electrochemical cell which has the most positive electrode potential.
potassium glass → kalijevo staklo
Potassium glass is a type of glass produced from potassium silicates and calcium with potassium carbonate. It dissolves harder than regular glass and it is used in production of chemical vessels.
Citing this page:
Generalic, Eni. "Kemijski potencijal." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table