decomposing → raščinjavanje
Decomposing in analytical chemistry means that a certain substance is converted, by melting it with a suitable melting medium (sodium carbonate, sodium hydroxide, sodium peroxide, ...) in the kind of compound which will afterwards that dissolve in water, acid or base very easily.
dehydrogenation → dehidrogenacija
Dehydrogenation is a chemical reaction in which hydrogen is removed from a compound. Dehydrogenation of organic compounds converts single carbon-carbon bonds into double bonds. It is usually affected by means of a metal catalyst or in biological systems by enzyme dehydrogenases.
detergent → deterdžent
Detergent is a substance added to water to improve its cleaning properties. Although water is a powerful solvent for many compounds, it will not dissolve grease and natural oils. Detergents are compounds that cause such nonpolar substances to go into solution in water. Soap is the original example, owing its action to the presence of ions formed from long-chain fatty acids ion (e.g. stearat ion, CH3(CH2)16COO-).
cellulose → celuloza
Cellulose, (C6H10O5)n, is a polysaccharide that consists of a long unbranched chain of glucose units linked by (1→4)-β-glycoside bonds. Nature uses cellulose primarily as a structural material to impart strength and rigidity to plants. Leaves, grasses, and cotton are primarily cellulose. The fibrous nature of extracted cellulose has led to its use in textile industry for the production of cotton, artificial silk, etc. Cellulose also serves as raw material for the manufacture of cellulose acetate, known commercially as acetate rayon, and cellulose nitrate, known as guncotton. Gunncotton is the major ingredient in smokeless powder, the explosive propellant used in artillery shells and in ammunition for firearms.
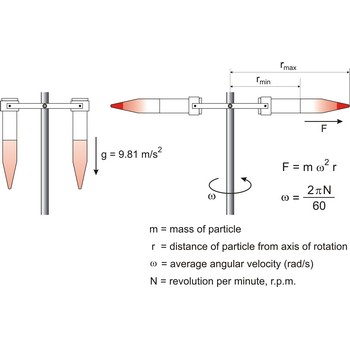
centrifuge → centrifuga
Centrifuge is a device in which solid or liquid particles of different densities are separated by rotating them in a tube in a horizontal circle. The dense particles tend to move along the length of the tube to a greater radius of rotation, displacing the lighter particles to the other end.
effervescence → pjenušanje
Effervescence is the formation of gas bubbles in a liquid by a chemical reaction. An example of effervescence is the release of carbon dioxide which bubbles as a gas from the liquid when limestone chips, which are composed of calcium carbonate, are added to dilute hydrochloric acid.
electron pair → elektronski par
Electron pair is two electrons within one orbital with opposite spins responsible for a chemical bond.
equivalent → ekvivalent
Equivalent (eq) is a unit for describing the amount of a chemical species. In contrast to the mole, the amount of a substance contained in one equivalent can vary from reaction to reaction.
Citing this page:
Generalic, Eni. "Kemijska ravnoteža." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table