boron → bor
Boron compounds have been known for thousands of years, but the element was not discovered until 1808 by Sir Humphry Davy (England) and independently by Joseph-Louis Gay-Lussac (France) and L. J. Thenard (France). The origin of the name comes from the Arabic word buraq and the Persian word burah meaning boraks. It is hard, brittle, lustrous black semimetal. Unreactive with oxygen, water, alkalis or acids. Combines with most metals to form borides. Boron is obtained from kernite, a kind of borax (Na2B4O7·10H2O). High purity boron is produced by electrolysis of molten potassium fluroborate and potassium chloride (KCl). Amorphous boron is used in pyrotechnic flares to provide a distinctive green color and in rockets as an igniter.
chemiluminescence → kemiluminiscencija
Chemiluminescence is energy release in form of electromagnetic radiation during a chemical reaction.
chemisorption → kemisorpcija
Chemisorption is a binding of a liquid or gas on the surface or in the interior of a solid by chemical bonds or forces.
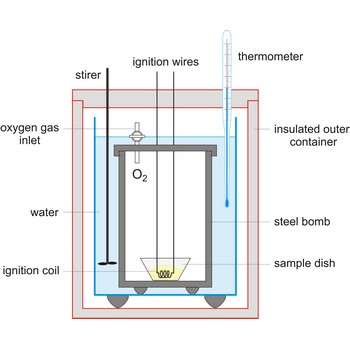
calorimeter → kalorimetar
Calorimeter is an instrument used to measure the energy absorbed or released in a chemical reaction. It also used in determining specific heat.
Carnot cycle → Carnotov kružni proces
Carnot cycle is the most efficient cycle of operations for a reversible heat engine. Published in 1824 by French physicist Nicolas Léonard Sadi Carnot (1796-1832), it consists of four operations on the working substance in the engine:
1-2: Isothermal expansion at thermodynamic temperature T1 with heat QH taken in.
2-3: Adiabatic expansion with a fall of temperature to T2.
3-4: Isothermal compression at temperature T2 with heat QC given out.
4-1: Adiabatic compression at temperature back to T1.
According to the Carnot principle, the efficiency of any reversible heat engine depends only on the temperature range through which it works, rather than the properties of the working substances.
catalyst → katalizator
Catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change. Catalysts that have the same phase as the reactants are homogenous catalysts (e.g. enzymes in biochemical reactions). Those that have a different phase are heterogeneous catalyst (e.g. metals or oxides used in gas reactions).
The catalyst provides an alternative pathway by which the reaction can proceed, in which the activation energy is lower. In thus increases the rate at which the reaction comes to an equilibrium, although it does not alter the position of the equilibrium.
chemotherapy → kemoterapija
Chemotherapy is the treatment of disease by means of chemicals that have a specific toxic effect upon the disease producing microorganisms (antibiotics) or that selectively destroy cancerous tissue (anticancer therapy).
computational chemistry → kompjutacijska kemija
Computational chemistry is a branch of chemistry concerned with the prediction or simulation of chemical properties, structures, or processes using numerical techniques.
conductometry → konduktometrija
Conductometry is a volumetric analytic method in which the end of titration (equivalent point) is defined by an electric conductivity appliance.
Citing this page:
Generalic, Eni. "Kemijska promjena." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table