Schiff base → Schiffova baza
Schiff base is a class of compounds derived by the chemical reaction (condensation) of aldehydes or ketones with aromatic amines, for example
They were named after the German chemist Hugo Schiff (1834-1915).
synthetic material → sintetički materijal
Synthetic material (artificial material) is a substance manufactured by chemical synthesis.
theories of catalysis → teorije katalize
Theories of catalysis explain the influence of the catalysts upon the rate of a reaction by describing the detailed mechanism by which the catalyst is involved in the steps of the chemical reaction.
thermit welding → termitno zavarivanje
Thermit welding is a group of welding processes in which fusion is produced by heating with superheated liquid metal resulting from a chemical reaction between a metal oxide and aluminium.
glucose → glukoza
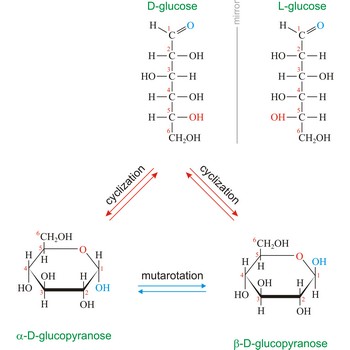
Glucose (grape sugar, blood sugar), C6H12O6, is an aldohexose (a monosaccharide sugar having six carbon atoms and an aldehyde group). An older common name for glucose is dextrose, after its dextrorotatory property of rotating plane polarized light to the right. Glucose in free (in sweet fruits and honey) or combined form (sucrose, starch, cellulose, glycogen) is is probably the most abundant organic compound in nature. During the photosynthesis process, plants use energy from the sun, water from the soil and carbon dioxide gas from the air to make glucose. In cellular respiration, glucose is ultimately broken down to yield carbon dioxide and water, and the energy from this process is stored as ATP molecules (36 molecules of ATP across all processes).
Naturally occurring glucose is D isomers (OH group on the stereogenic carbon farthest from the aldehyde group, C-5, is to the right in the Fischer projection). Although often displayed as an open chain structure, glucose and most common sugars exist as ring structures. In the α form, the hydroxyl group attached to C-1 and the CH2OH attached to C-5 are located on opposite sides of the ring. β-glucose has these two groups on the same side of the ring. The full names for these two anomers of glucose are α-D-glucopyranose and β-D-glucopyranose.
haematite → hematit
Haematite is a mineral of iron(III) oxide Fe2O3. It is the most important ore of iron and usually occurs in two main forms: as a massive red kidney-shaped ore and as grey to black metallic crystals known as specular iron ore. Haematite is the major red colouring agent in rocks; the largest deposits are of sedimentary origin. In industry haematite is also used as a polishing agent (jeweller’s rouge) and in paints.
thermochemistry → termokemija
Termochemistry is the study of heat absorbed or released during chemical changes.
thermodynamics → termodinamika
Thermodynamics is the scientific study of the interconversion of heat and other forms of energy.
toxic chemical → bojni otrov
Toxic chemical means any chemical which through its chemical action on life processes can cause death, temporary incapacitation, or permanent harm to humans or animals. This includes all such chemicals, regardless of their origin or of their method of production, and regardless of whether they are produced in facilities, in munitions or elsewhere.
valence bond → valentna veza
In the valence bond theory, a valence bond is a chemical bond formed by overlap of half-filled atomic orbitals on two different atoms.
Citing this page:
Generalic, Eni. "Kemijska kinetika." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table