aromatic compounds → aromatski ugljikovodici
Aromatic compounds are a major group of unsaturated cyclic hydrocarbons containing one or more rings, typified by benzene, which has a 6-carbon ring containing three double bonds. All the bonds in benzene (C6H6) are the same length intermediate between double and single C-C bonds. The properties arise because the electrons in the p-orbitals are delocalised over the ring, giving extra stabilization energy of 150 kJ/mol over the energy of Kekulé structure. Aromatic compounds are unsaturated compounds, yet they do not easily partake in addition reactions.
Historical use of the term implies a ring containing only carbon (e.g., benzene, naphthalene), but it is often generalized to include heterocyclic structures such as pyridine and thiophene.
asbestos → azbest
Asbestos is any one of a group of fibrous amphibole minerals (from the silicate group). It has widespread commercial uses because of its resistance to heat, chemical inertness., and high electrical resistance. Since 1970s short asbestos fibres have been recognized as a cause of asbestosis, a serious lung disorder, and mesothelioma, a fatal form of lung cancer. These concerns have limited its use and imposed many safety procedures when it is used.
astatine → astat
Astatine was discovered by Emilio Gino Segrè, Dale R. Corson and K. R. MacKenzie (USA) in 1940. The origin of the name comes from the Greek word astatos meaning unstable. It is unstable, radioactive member of the halogen group. Astatine does not occur in nature. Similar to iodine. Produced by bombarding bismuth with alpha particles. Since its isotopes have such short half-lives there are no commercially significant compounds of astatine.
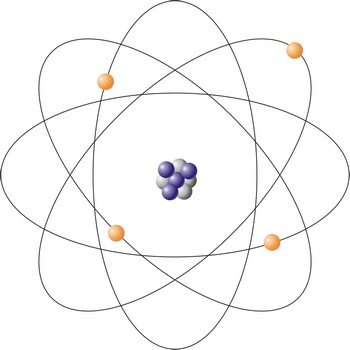
atom → atom
Atom is an atom is the smallest particle of an element that retains the chemical properties of the element. Rutherford-Bohr’s model represents the atom as a positively charged core of a size around 10-14 m composed of protons (positive particles) and neutrons (neutral particles) around which negatively charged electrons circle. The number of protons and electrons are equal, so the atom is an electrically a neutral particle. Diameter of the atom is about 10-10 m.
base → baza
Historically, base is a substance that yields an OH - ion when it dissociates in solution, resulting in a pH>7. In the Brønsted definition, a base is a substance capable of accepting a proton in any type of reaction. The more general definition, due to G.N. Lewis, classifies any chemical species capable of donating an electron pair as a base. Typically, bases are metal oxides, hydroxides, or compounds (such as ammonia) that give hydroxide ions in aqueous solution.
battery → baterija
Battery a device that converts chemical energy to electrical energy. The process underlying the operation of a battery involves a chemical reaction in which electrons are transferred from one chemical species to another. This process is carried out in two half-reactions, one that involves the loss of electrons and one that involves their gain. The battery is an electrochemical cell divided in two half-cells, and reaction proceeds when these are connected together by an electrically conducting pathway. The passage of electrons from one half-cell to the other corresponds to an electric current. Each half-cell contains an electrode in contact with the reacting species. The electrode which passes electrons into the circuit when battery discharges is called anode and is negative terminal. The electrode which receives electrons is called cathode, and is the battery’s positive terminal. The electrical circuit is completed by an electrolyte, an electrically conducting substance placed between the two electrodes which carriers a flow of charge between them. In wet cells, the electrolyte is a liquid containing dissolved ions, whose motion generates an electrical current; in dry cells the electrolyte is basely solid, for example, a solid with mobile ions or porous solid saturated with an ionic solution.
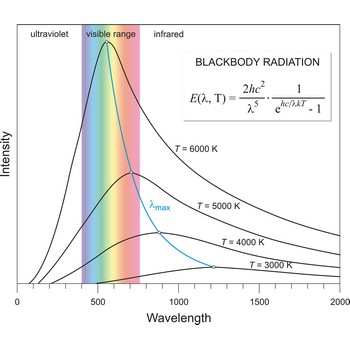
blackbody radiation → zračenje crnog tijela
Blackbody radiation is the radiation emitted by a perfect blackbody, i.e., a body which absorbs all radiation incident on it and reflects none. The primary law governing blackbody radiation is the Planck Radiation Law, which governs the intensity of radiation emitted by unit surface area into a fixed direction (solid angle) from the blackbody as a function of wavelength for a fixed temperature. The Planck Law can be expressed through the following equation
where λ is the wavelength, h is Planck’s constant, c is the speed of light, k is the Boltzmann constant, and T is the temperature.
boron → bor
Boron compounds have been known for thousands of years, but the element was not discovered until 1808 by Sir Humphry Davy (England) and independently by Joseph-Louis Gay-Lussac (France) and L. J. Thenard (France). The origin of the name comes from the Arabic word buraq and the Persian word burah meaning boraks. It is hard, brittle, lustrous black semimetal. Unreactive with oxygen, water, alkalis or acids. Combines with most metals to form borides. Boron is obtained from kernite, a kind of borax (Na2B4O7·10H2O). High purity boron is produced by electrolysis of molten potassium fluroborate and potassium chloride (KCl). Amorphous boron is used in pyrotechnic flares to provide a distinctive green color and in rockets as an igniter.
Bragg angle → Braggov kut
Bragg angle (Θ) is the angle between an incident X-ray beam and a set of crystal planes for which the secondary radiation displays maximum intensity as a result of constructive interference. British physicist Sir William Henry Bragg and his son Sir William Lawrence Bragg developed a simple relation for scattering angles, now call Bragg’s law.
which relates the angle θ between a crystal plane and the diffracted X-ray beam, the wavelength λ of the x-rays, the crystal plane spacing d, and the diffraction order n (any integer).
The diffraction experiment as presently considered is intended to provide quantitative information on the lattice constant and shape characteristics of the unit cell.
Born-Haber cycle → Born-Haberov kružni proces
Born-Haber cycle is a cycle of reactions used for calculating the lattice energies of ionic crystalline solids. For a compound MX, the lattice energy is the enthalpy of the reaction
The standard enthalpy of formation of the ionic solid is the enthalpy of the reaction
The cycle involves equating this enthalpy (which can be measured) to the sum of the enthalpies of a number of steps proceeding from the elements to the ionic solid. The steps are:
1) Atomization of the metal
2) Atomization of the nonmetal
3) Ionisation of the metal
This is obtained from the ionisation potential.
4) Ionisation of the nonmetal
This is electron affinity.
5) Formation of the ionic solids
Equation of the enthalpies gives
from which ΔHL can be found.
Citing this page:
Generalic, Eni. "Kemijska jednadžba." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table