aqueous solution → vodena otopina
Aqueous solutions are those solutions where water is the solvent. An aqueous solution found in an equation describing a chemical reaction is denoted by the state symbol, (aq).
equilibrium constant → konstanta ravnoteže
The equilibrium constant (K) was originally introduced in 1863 by Norwegian chemists C.M. Guldberg and P. Waage using the law of mass action. For a reversible chemical reaction represented by the equation
chemical equilibrium occurs when the rate of the forward reaction equals the rate of the back reaction, so that the concentrations of products and reactants reach steady-state values.
The equilibrium constant is the ratio of chemical activities of the species A, B, C, and D at equilibrium.
To a certain approximation, the activities can be replaced by concentrations.
For gas reactions, partial pressures are used rather than concentrations
The units of Kp and Kc depend on the numbers of molecules appearing in the stoichiometric equation (a, b, c, and d).
The value equilibrium constant depends on the temperature. If the forward reaction is exothermic, the equilibrium constant decreases as the temperature rises. The equilibrium constant shows the position of equilibrium. A low value of K indicates that [C] and [D] are small compared to [A] and [B]; i.e. that the back reaction predominates.
The equilibrium constant is related to ΔrG°, the standard Gibbs free energy change in the reaction, by
Gibbs free energy → Gibbsova slobodna energija
Gibbs free energy (G) is an important function in chemical thermodynamics, defined by
where H is the enthalpy, S the entropy, and T the thermodynamic temperature. Gibbs free energy is the energy liberated or absorbed in a reversible process at constant pressure and constant temperature. Sometimes called Gibbs energy and, in older literature, simply free energy.
Changes in Gibbs free energy, ΔG, are useful in indicating the conditions under which a chemical reaction will occur. If ΔG is negative the reaction will proceed spontaneously to equilibrium. In equilibrium position ΔG = 0.
stoichiometric coefficient → stehiometrijski koeficijent
Stoichiometric coefficient (ν) is the number appearing before the symbol for each compound in the equation for a chemical reaction. By convention, it is negative for reactants and positive for products.
Stoichiometric coefficients describe the stoichiometry of the chemical reaction.
In this equation, a, b, c and d are called as Stoichiometric coefficients of the A, B, C and D respectively.
stoichiometry → stehiometrija
Stoichiometry is the relative proportions elements from compounds or in which substances react. Every chemical reaction has its characteristic proportions. For example, when methane unites with oxygen in complete combustion, 1 mol of methane requires 2 mol of oxygen.
At the same time, 1 mol of carbon dioxide and 2 mol of water are formed as reaction products.
Alternatively, 16 g of methane and 64 g of oxygen produce 44 g of carbon dioxide and 36 g of water.
The stoichiometric relationship between the products and reactants can be used to in calculations.
accelerator → akcelerator
Accelerator is a device (machine) used for acceleration of charged particles (protons, deuterons, α-particles). Particles are accelerated under the influence of an electric field and with the help of a magnetic field are kept inside a certain space. When the particles reach enough acceleration (that is sufficient energy), they are directed on a target we wish to bomb. Best known types cyclotron, synchrotron, betatron.
Accelerator is a substance that increases the rate of chemical reaction, i.e. a catalyst.
accumulator → akumulator
Accumulator (secondary cell, storage battery) is a type of voltaic cell or battery that can be recharged by passing current through it from an external D.C. supply. The charging current reverses the chemical reactions in the cell. The common types are the lead-acid accumulator and the nickel-cadmium cell.
activated charcoal → aktivni ugljen
Activated charcoal or activated carbon is charcoal that has been activated for adsorption by steaming or by heating in a vacuum. Charcoal is obtained by burning wood, nutshells, coconut husks or other materials. Charcoal becomes activated by heating it with steam to approximately 1000 °C in the absence of oxygen.
The chemical nature of amorphous carbon, combined with a high surface area makes it an ideal medium for the adsorption of organic chemicals. A single gram of such material can have 400 m2 to 1 200 m2 square meters of surface area. Activated charcoal is widely used to decolorize liquids, recover solvents, and remove toxins from water and air.
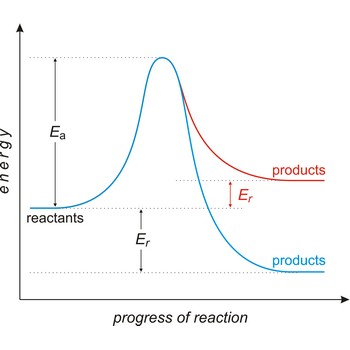
activation energy → energija aktivacije
Activation energy (Ea) is the energy that must be added to a system in order for a process to occur, even though the process may already be thermodynamically possible. In chemical kinetics, the activation energy is the height of the potential barrier separating the products and reactants. It determines the temperature dependence on the reaction rate.
Citing this page:
Generalic, Eni. "Kemijska jednadžba." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table