iridium → iridij
Iridium was discovered by Smithson Tennant (England) in 1803. The origin of the name comes from the Latin word iris, meaning rainbow, because its salts are highly colored. It is heavy, brittle, white metal. Unreactive in air, water and acids. Attacked by fused NaOH. Metal ignites and burns readily. Iridium is found in gravel deposits with platinum. Used with osmium to tip gold pen points, to make crucible and special containers. Also to make alloys used for standard weights and measures and heat-resistant alloys. Also as hardening agent for platinum.
metal → metal
Metals are materials in which the highest occupied energy band (conduction band) is only partially filled with electrons.
Their physical properties generally include:
- They are good conductors of heat and electricity. The electrical conductivity of metals generally decreases with temperature.
- They are malleable and ductile in their solid state.
- They show metallic lustre.
- They are opaque.
- They have high density.
- They are solids (except mercury)
- They have a crystal structure in which each atom is surrounded by eight to twelve near neighbours
Their chemical properties generally are:
- They have one to four valence electrons.
- They have low ionisation potentials; they readily lose electrons.
- They are good reducing agents.
- They have hydroxides which are bases or amphoteric.
- They are electropositive.
Metallic characteristics of the elements decrease and non-metallic characteristics increase with the increase of valence electrons. Also metallic characteristics increase with the number of electron shells. Therefore, there is no sharp dividing line between the metals and non-metals.
Of the 114 elements now known, only 17 show primarily non-metallic characteristics, 7 others are metalloids, and 89 may be classed as metals.
nerve poison → živčani bojni otrov
Nerve poison (nerve gas, agents) have had an entirely dominant role since the Second World War. Nerve poisons acquired their name because they affect the transmission of nerve impulses in the nervous system. All nerve poisons belong chemically to the group of organo-phosphorus compounds. They are stable and easily dispersed, highly toxic and have rapid effects both when absorbed through the skin and via respiration. Nerve poisons can be manufactured by means of fairly simple chemical techniques. The raw materials are inexpensive and generally readily available.
The most important nerve agents included in modern chemical weapons arsenals are:
| Tabun | (o-ethyl dimethylamidophosphorylcyanide) |
| Sarin | (isopropyl methylphosphonofluoridate) |
| Soman | (pinacolyl methylphosphonofluoridate) |
| GF | (cyclohexyl methylphosphonofluoridate) |
| VX | (o-ethyl S-diisopropylaminomethyl methylphosphonothiolate) |
Nerve poisons are colorless, odorless, tasteless liquids of low volatility. Antidotes are atropine sulfate and pralidoxime iodide.
non-metal → nemetal
Non-metals are defined as elements that are not metals.
Their physical properties generally include:
- They are poor conductors.
- They are brittle, not ductile in their solid state.
- They show no metallic lustre.
- They may be transparent or translucent.
- They have low density.
- They form molecules which consists of atoms covalently bonded; the noble gases are monoatomic.
Their chemical properties are generally:
- They usually have four to eight valence electrons.
- They have high electron affinities (except the noble gases)
- They are good oxidising agents (except the noble gases)
- They have hydroxides which are acidic (except the noble gases)
- They are electronegative.
redox titration → redoks-titracija
Redox titration (oxidation-reduction titration) is a titration based on a redox reaction. For example, iron in water can be determined by converting dissolved iron to Fe2+ and titrating the solution with potassium permanganate (KMnO4), a powerful oxidising agent.
rhenium → renij
Rhenium was discovered by Walter Noddack, Ida Tacke and Otto Berg (Germany) in 1925. The origin of the name comes from the Latin word Rhenus meaning river Rhine. It is rare and costly, dense, silvery-white metal. Tarnishes in moist air. Resists corrosion and oxidation. Dissolves in nitric and sulfuric acids. Has a very high melting point. Rhenium is found in small amounts in gadolinite and molybdenite. Mixed with tungsten or platinum to make filaments for mass spectrographs. Its main value is as a trace alloying agent for hardening metal components that are subjected to continuous frictional forces.
sediment → sediment
1. Sediment is a fragmental material that originates from weathering of rocks and is transported by, suspended in, or deposited by water or air or is accumulated in beds by other natural agencies. When solidified, sediments form sedimentary rocks.
2. Strictly, sediment is a solid material that has settled down from a state of suspension in a liquid.
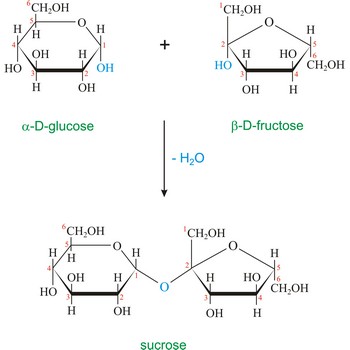
sucrose → saharoza
Sucrose (saccharose), or ordinary table sugar, is a disaccharide in which α-D-glucopyranose and β-D-fructofuranose are joined at their anomeric carbons by a glycosidic bond. There are no hemiacetals remaining in the sucrose and therefore sucrose is not a reducing sugar and does not exhibit mutarotation. Sugar is a white crystalline sweet compound found in many plants and extracted from sugar cane and sugar beet. It is used as a sweetening agent in food and drinks. If heated to 200 °C, sucrose becomes caramel. When sucrose is hydrolyzed it forms an equimolar mixture of glucose and fructose. This mixture of monosaccharides is called invert sugar. Honeybees have enzymes called invertases that catalyze the hydrolysis of sucrose. Honey, in fact, is primarily a mixture of glucose, fructose, and sucrose.
superoxide → superoksid
Superoxides are binary compounds containing oxygen in the -½ oxidation state. Sodium superoxide (NaO2) can be prepared with high oxygen pressures, whereas the superoxides of rubidium, potassium, and cesium can be prepared directly by combustion in air. These compounds are yellow to orange paramagnetic solids. Superoxide ion, O2-, has an unpaired electron, is not particularly stable, and spontaneously decomposes into peroxide over time.
They are strong oxidising agents that vigorously hydrolyze (react with water) to produce superoxide and oxygen gas.
Citing this page:
Generalic, Eni. "Kelatni agens." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table