electroanalytical chemistry → elektroanalitička kemija
Electroanalytical chemistry chemistry is the application of electrochemical cells and electrochemical techniques for chemical analysis. The analyte is dissolved in the electrolyte of the cell, and one can perform either qualitative analysis (determination of the type of constituents present) or quantitative analysis (determination of the amount of a given constituent).
equivalent → ekvivalent
Equivalent (eq) is a unit for describing the amount of a chemical species. In contrast to the mole, the amount of a substance contained in one equivalent can vary from reaction to reaction.
cross-linking → umrežavanje
Cross-linking is an attachment of two chains of polymer molecules by bridges, composed of either an element, a group, or a compound, that join certain carbon atoms of the chains by primary chemical bonds, as indicated in the schematic diagram
Cross-linking occurs in nature in substances made up of polypeptide chains that are joined by the disulfide bonds of the cysteine residue, as in keratins or insulin. Cross-linking can be artificially effected, either adding a chemical substance (cross-linking agent), or by subjecting the polymer to high-energy radiation. Examples are: vulcanisation of rubber with sulphur, cross-linking of polystyrene with divinylbenzene, or cross-linking of polyethylene by means of high-energy radiation.
Cross-linking has the effect of changing a plastic from thermoplastic to thermosetting. Thus, it also increases strength, heat and electrical resistance, and especially resistance to solvents and other chemicals.
crucible → lončić za žarenje
Crucible is used for heating small amounts of solid in an oven to very high temperatures. Crucibles are usually made out of porcelain, platinum, nickel or iron.
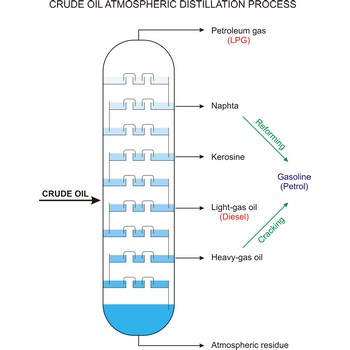
crude oil → sirova nafta
Crude oil (petroleum) is a fossil fuel formed from plant and animal remains many million of years ago. It is occasionally found in springs or pools but is usually drilled from wells beneath the earth’s surface. Crude oil is a mixture of hydrocarbons with small quantities of other chemicals such as sulphur, nitrogen and oxygen. Crude is the raw material which is refined into petrol, heating oil, jet fuel, propane, petrochemicals, and other products.
density → gustoća
In the most common usage, density (ρ) is mass density or mass per unit volume. In Si units it is measured in kg m-3. More commonly, densities are given in kg dm-3.
More generally, it is the amount of some quantity (mass, charge, energy, etc.) divided by a length, area, or volume.
Relative density is the ratio of the density of a substance to the density of some reference substance. For liquids or solids, it is the ratio of the density (usually at 20 °C) to the density of water at 4 °C. This quantity was formerly called specific gravity.
equivalent weight → ekvivalentna masa
Equivalent weight of a substance participating in a neutralization reaction is that mass of substance (molecule, ion, or paired ion) that either reacts with or supplies 1 mol of hydrogen ions in that reaction.
Equivalent weight of a substance participating in an oxidation/reduction reaction is that weight which directly or indirectly produces or consumes 1 mol of electrons.
eutectic → eutektik
Eutectic is a solid solution consisting of two or more substances and having the lowest freezing point of any possible mixture of these components.
Eutectic point is the lowest temperature at which the eutectic mixture can exist in a liquid phase. A liquid having the eutectic composition will freeze at a single temperature without a change of composition.
crystal system → kristalni sustav
Crystal system is a method of classifying crystalline substances on the basis of their unit cell. There are seven unique crystal systems. The simplest and most symmetric, the cubic (or isometric) system, has the symmetry of a cube. The other six systems, in order of decreasing symmetry, are hexagonal, tetragonal, rhombohedral (also known as trigonal), orthorhombic, monoclinic and triclinic.
|
Crystal system
|
Unit-cell
|
Conditions on unit-cell edges and angles |
|
cubic |
 |
a=b=c α=β=γ=90° |
|
hexagonal |
 |
a≠c α=γ=90° β=120° |
|
tetragonal |
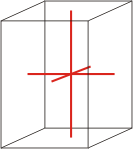 |
a=b≠c α=β=γ=90° |
|
rhombohedral |
 |
a=b=c α=β=γ≠90° |
|
orthorhombic |
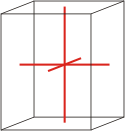 |
a≠b≠c α=β=γ=90° |
|
monoclinic |
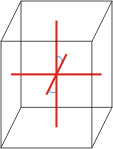 |
a≠b≠c α=γ=90°≠β |
|
triclinic |
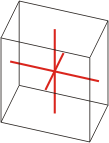 |
a≠b≠c α≠β≠γ≠90° |
Citing this page:
Generalic, Eni. "Kancerogene tvari." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table