aqueous solution → vodena otopina
Aqueous solutions are those solutions where water is the solvent. An aqueous solution found in an equation describing a chemical reaction is denoted by the state symbol, (aq).
Archimedes law → Arhimedov zakon
The upward force (buoyancy force) is exerted on a body floating in a fluid. It equals the weight of the displaced fluid.
Avogadro, Amadeo → Avogadro, Amadeo
Amadeo Avogadro (1776-1856) is an Italian chemist and physicist that proposed a correct molecular explanation for Gay-Lussac’s law of combining volumes. His work provided a simple way to determine atomic weights and molecular weights of gases. He is published a theory about the movement of particles in gases that became known as Avogadro’s Law.
Avogadro’s law → Avogadrov zakon
Avogadro’s law: Equal volumes of all gases contain equal numbers of molecules at the same pressure and temperature. The law, often called Avogadro’s hypothesis, is true only for ideal gases. It was proposed in 1811 by Italian chemist Amadeo Avogadro (1776-1856).
blackbody → crno tijelo
In radiation physics, an ideal blackbody is a theoretical object that absorbs all the radiant energy falling upon it and emits it in the form of thermal radiation. Planck’s radiation law gives the power radiated by a unit area of blackbody, and the Stefan-Boltzman law expresses the total power radiated.
argon → argon
Argon was discovered by Lord Raleigh and Sir William Ramsay (Scotland) in 1894. The origin of the name comes from the Greek word argos meaning inactive. It is colourless and odourless noble gas. Chemically inert. It is the third most abundant element in the earth’s atmosphere and makes up about 1 %. Argon is continuously released into the air by decay of radioactive potassium-40. Pure form is obtained from fractional distillation of liquid air. Used in lighting products. It is often used in filling incandescent light bulbs. Some is mixed with krypton in fluorescent lamps. Crystals in the semiconductor industry are grown in argon atmospheres.
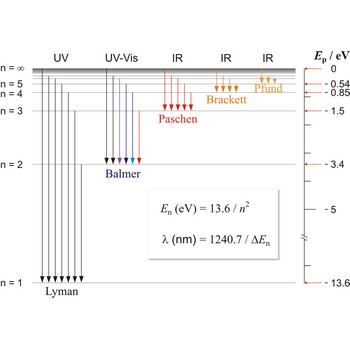
Balmer series → Balmerova serija
Balmer series, Balmer lines is a series of lines in the emission spectrum of hydrogen that involve transitions to the n=2 state from states with n>2.
Bohr atom → Bohrov atom
Bohr atom is a model of the atom that explains emission and absorption of radiation as transitions between stationary electronic states in which the electron orbits the nucleus at a definite distance. The Bohr model violates the Heisenberg uncertainty principle since it postulates definite paths and moment for electrons as they move around the nucleus. Modern theories usually use atomic orbitals to describe the behaviour of electrons in atoms.
Boltzmann constant → Boltzmannova konstanta
The Boltzmann constant (k or kB) is the physical constant describing the relationship between the thermodynamic temperature and the average kinetic energy of particles in a gas. It equals the molar gas constant R divided by the Avogadro constant NA and has the value 1.380 648 52(79)×10-23 J/K. It is named after the Austrian physicist Ludwig Eduard Boltzmann (1844-1906).
Born-Haber cycle → Born-Haberov kružni proces
Born-Haber cycle is a cycle of reactions used for calculating the lattice energies of ionic crystalline solids. For a compound MX, the lattice energy is the enthalpy of the reaction
The standard enthalpy of formation of the ionic solid is the enthalpy of the reaction
The cycle involves equating this enthalpy (which can be measured) to the sum of the enthalpies of a number of steps proceeding from the elements to the ionic solid. The steps are:
1) Atomization of the metal
2) Atomization of the nonmetal
3) Ionisation of the metal
This is obtained from the ionisation potential.
4) Ionisation of the nonmetal
This is electron affinity.
5) Formation of the ionic solids
Equation of the enthalpies gives
from which ΔHL can be found.
Citing this page:
Generalic, Eni. "Jednadžba stanja idealnog plina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table