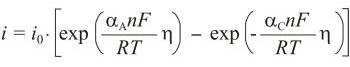Butler-Volmer equation → Butler-Volmerova jednadžba
Butler-Volmer equation is an activation controlled reaction, the one for which the rate of reaction is controlled solely by the rate of the electrochemical charge transfer process, which is in turn an activation-controlled process. This gives rise to kinetics that are described by the Butler-Volmer equation:
where io is exchange current density, η is overpotential (η = E - Eo), n is number of electrons, αA is anodic transfer coefficient, and αC is cathodic transfer coefficient
change of state → promjena stanja
Change of state is a physical change which appears when a substance crosses from one state into another. This usually happens because of the change of energy of particles provoked by heating or cooling.
complete ionic equation → potpuna ionska jednadžba
Complete ionic equation is a balanced equation that describes a reaction occurring in a solution, in which all strong electrolytes are written as dissociated ions.
chemical reaction equation → jednadžba kemijske reakcije
Chemical reactions are symbolically shown with chemical equations. On the left side of the equation we write formulas or substance symbols which enter the chemical reaction, reactants. On the right side formulas or substance symbols which emerge from the chemical reaction, products, are writen.
Each chemical reaction leads to an equilibrium which is moved more or less to one side (left or right). Because of that, in reversible reactions instead of = sign two opposite arrows are put
In order to write down certain chemical reaction equation all reactants and all products and their stechiometric proportions must be known. (See Chemical reaction balancing)
Clapeyron equation → Clapeyronova jednadžba
Clapeyron equation (also called the Clausius-Clapeyron equation) is a relation between pressure and temperature of two phases of a pure substance that are in equilibrium,
where ΔtrsS is the difference in entropy between the phases and ΔtrsV the corresponding difference in volume.
Einstein equation → Einsteinova jednadžba
Einstein equation is the mass-energy relationship introduced by Albert Einstein in 1905 in the form E = mc2, where E is a quantity of energy, m its mass, and c is the speed of light. It presents the concept that energy possesses mass.
rate equation → jednadžba brzine reakcije
Rate equation is an equation that describes the dependence of reaction rate on concentrations of reacting species. It always has the form
where a and b are usually integral exponents.
Ilkovic equation → Ilkovičeva jednadžba
Ilkovic equation is a relation used in polarography relating the diffusion current (id) and the concentration of the depolarizer (c), which is the substance reduced or oxidized at the dropping mercury electrode. The Ilkovic equation has the form
Where k is a constant which includes Faraday constant, π and the density of mercury, and has been evaluated at 708 for max current and 607 for average current, D is the diffusion coefficient of the depolarizer in the medium (cm2/s), n is the number of electrons exchanged in the electrode reaction, m is the mass flow rate of Hg through the capillary (mg/sec), and t is the drop lifetime in seconds, and c is depolarizer concentration in mol/cm3.
The equation is named after the scientist who derived it, the Slovak chemist, Dionýz Ilkovič 1907-1980).
Schrodinger equation → Schrodingerova jednadžba
Schrödinger equation is the basic equation of wave mechanics which, for systems not dependent on time, takes the form:
where Ψ is the wavefunction, V is the potential energy expressed as a function of the spatial coordinates, E its total energy, ![]() 2 is the Laplacian operator, h is Planck’s constant, and m is the mass.
2 is the Laplacian operator, h is Planck’s constant, and m is the mass.
ideal gas → idealni plin
Ideal gas is a gas in which there is complete absence of cohesive forces between the component molecules; the behaviour of such a gas can be predicted accurately by the ideal gas equation through all ranges of temperature and pressure. The concept is theoretical, since no actual gas meets the ideal requirement.
Citing this page:
Generalic, Eni. "Jednadžba stanja idealnog plina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table