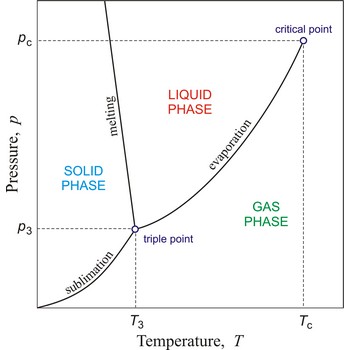
critical point → kritična točka
In general, critical point is the point on the phase diagram of a two-phase system at which the two coexisting phases have identical properties and therefore represent a single phase. At the liquid-gas critical point of a pure substance, the distinction between liquid and gas vanishes, and the vapour pressure curve ends. The coordinates of this point are called the critical temperature and critical pressure. Above the critical temperature it is not possible to liquefy the substance.
freezing → smrzavanje
Freezing is the change of a liquid into a solid state as the temperature decreases. For water, the freezing point is 0 °C (or 273.16 K).
freezing point → ledište
Freezing point is the temperature at which a liquid becomes a solid at normal atmospheric pressure.
See Melting point
glass transition temperature → temperatura staklastog prijelaza
Glass transition temperature (Tg) is the temperature at which an amorphous polymer is transformed, in a reversible way, from a viscous or rubbery condition to a hard and relatively brittle one.
gravitational constant → gravitacijska konstanta
Gravitational constant (G) is the universal constant in the equation for the gravitational force between two particles
where r is the distance between the particles and m1 and m2 are their masses.
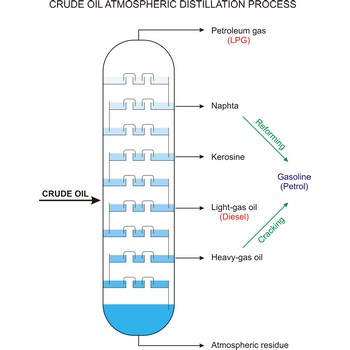
crude oil → sirova nafta
Crude oil (petroleum) is a fossil fuel formed from plant and animal remains many million of years ago. It is occasionally found in springs or pools but is usually drilled from wells beneath the earth’s surface. Crude oil is a mixture of hydrocarbons with small quantities of other chemicals such as sulphur, nitrogen and oxygen. Crude is the raw material which is refined into petrol, heating oil, jet fuel, propane, petrochemicals, and other products.
Dalton’s law → Daltonov zakon
Dalton’s law of partial pressure says that the total pressure eof gaseous mixture is equal to the sum of all gases partial pressures which make that mixture on the condition that they do not interact.
For example, if dry oxygen gas at 900 hPa is saturated with water vapor at 56 hPa, the pressure of the wet gas is 956 hPa.
heat of fusion → toplina taljenja
Heat of fusion or enthalpy of fusion is the heat required to convert a substance from the solid to the liquid state with no temperature change (also called latent heat of fusion or melting).
heat of reaction → toplina kemijske reakcije
Heat of reaction or enthalpy of reaction is the heat evolved or absorbed as a result of the complete chemical reaction of molar amounts of the reactants.
Citing this page:
Generalic, Eni. "Jednadžba stanja idealnog plina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table