redox reaction → redoks-reakcija
Redox reaction is an oxidation-reduction reaction is a reaction in which one or more electrons are transferred. When an atom, ion, or molecule loses one or more electrons, it is oxidised. When an atom, ion, or molecule gains one or more electrons, it is reduced.
velocity → brzina
If a point-like object moves so that its position vector changes from being ri to rf, than the displacement Δr of object is
If a point-like object undergoes a displacement, Δr, in time Δt, its average velocity, v is defined as
The instantaneous velocity, v, is obtained from the average velocity by shrinking the time interval Δt towards zero. The average velocity approaches a limiting value, which is the velocity of a given instant:
Velocity is a vector quantity. If we plot the path of a moving particle as a curve in a coordinate system, the instantaneous velocity is always tangent to that curve.
SI unit for velocity is m s-1.
accelerator → akcelerator
Accelerator is a device (machine) used for acceleration of charged particles (protons, deuterons, α-particles). Particles are accelerated under the influence of an electric field and with the help of a magnetic field are kept inside a certain space. When the particles reach enough acceleration (that is sufficient energy), they are directed on a target we wish to bomb. Best known types cyclotron, synchrotron, betatron.
Accelerator is a substance that increases the rate of chemical reaction, i.e. a catalyst.
aqueous solution → vodena otopina
Aqueous solutions are those solutions where water is the solvent. An aqueous solution found in an equation describing a chemical reaction is denoted by the state symbol, (aq).
chemical balance → kemijska ravnoteža
Chemical balance is a degree of reversible reaction in a closed system, when the forward and backward reaction happen at same rates and their effects annul each other, while the concentration of reactants and products stays the same.
chemical kinetics → kemijska kinetika
Chemical kinetics is an area of chemistry that studies rates and mechanisms of chemical reactions.
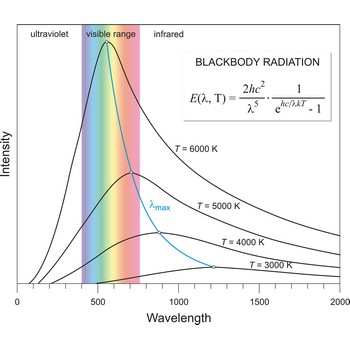
blackbody radiation → zračenje crnog tijela
Blackbody radiation is the radiation emitted by a perfect blackbody, i.e., a body which absorbs all radiation incident on it and reflects none. The primary law governing blackbody radiation is the Planck Radiation Law, which governs the intensity of radiation emitted by unit surface area into a fixed direction (solid angle) from the blackbody as a function of wavelength for a fixed temperature. The Planck Law can be expressed through the following equation
where λ is the wavelength, h is Planck’s constant, c is the speed of light, k is the Boltzmann constant, and T is the temperature.
law of chemical equilibrium → zakon o kemijskoj ravnoteži
Law of chemical equilibrium (also called the law of mass action) states that the rate at which a substance reacts is proportional to its active mass (i.e. to its molar concentration). Thus, the velocity of a chemical reaction is proportional to the product of the concentration of the reactants.
collision theory → teorija sudara
Collision theory is theory that explains how chemical reactions take place and why rates of reaction alter. For a reaction to occur the reactant particles must collide. Only a certain fraction of the total collisions cause chemical change; these are called successful collisions. The successful collisions have sufficient energy (activation energy) at the moment of impact to break the existing bonds and form new bonds, resulting in the products of the reaction. Increasing the concentration of the reactants and raising the temperature bring about more collisions and therefore more successful collisions, increasing the rate of reaction.
conditional electrode potential → uvjetni elektrodni potencijal
Conditional or formal electrode potential (E°’) is equal to electrode potential (E) when overall concentrations of oxidised and reduced form in all its forms in a solution are equal to one. Conditional electrode potential includes all effects made by reactions that do not take part in the electron exchange, but lead to change of ion power, changes of pH, hydrolysis, complexing, precipitating, etc.
At 298 K (25 °C) and by converting natural (Napierian) logarithms into decimal (common, or Briggian) logarithms, Nernst’s equation for electrode potential can be written as follows:
Citing this page:
Generalic, Eni. "Jednadžba brzine reakcije." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table