solution → otopina
Solutions are homogeneous mixtures of clean substances. Solutions contain two or more substances mixed in a state of molecular dispersion. Component which is found in solution in greater amount than other components is called a solvent and other components are called dissolved substances. Solution can be unsaturated, saturated and oversaturated.
supersaturated solution → prezasićena otopina
Solution is supersaturated when it contains greater quantity of dissolved substance in itself than it corresponds to solubility of that substance at that temperature. It is said to be in an unstable state, and by shaking the vessel containing that such a solution separation of salt surplus can occur.
unsaturated solution → nezasićena otopina
Unsaturated solution is a solution that contains less than the maximum possible equilibrium concentration of a solute.
ideal solution → idealna otopina
Ideal solution is a solution in which solvent-solvent and solvent-solute interactions are identical, so that properties such as volume and enthalpy are exactly additive. Ideal solutions follow Raoult’s law, which states that the vapour pressure pi of component i is pi = xi pi*, where xi is the mole fraction of component i and pi* the vapour pressure of the pure substance i.
ion-product constant → ionski produkt vode
The ion-product constant. For the reaction:
the equilibrium expression would be:
Note that all pure liquid terms are omitted, hence H2O does not appear in the denominator. At 25 °C
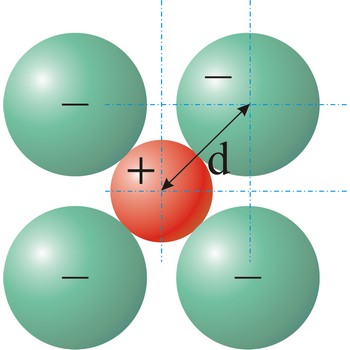
ionic radius → ionski radijus
Ionic radius is the radius of anions and cations in crystalline ionic compounds, as determined by consistently partitioning the center-to-center distance of ions in those compounds. In general, negative ions have larger ionic radii than positive ions.
saturated solution → zasićena otopina
Saturated solution is a solution that holds the maximum possible amount of dissolved material. When saturated, the rate of dissolving solid and that of recrystallisation solid are the same, and a condition of equilibrium is reached. The amount of material in solution varies with temperature; cold solutions can hold less dissolved solid material than hot solutions. Gases are more soluble in cold liquids than in hot liquids.
water ion product → ionski produkt vode
Water ion product (Kw) is a concentration product of hydrogen and hydroxide ions. For the reaction:
the equilibrium expression would be:
Note that all pure liquid terms are omitted, hence H2O does not appear in the denominator. At 25 °C, Kw = 1.0×10-14 mol2dm-6 = (Ka)(Kb)
acid → kiselina
Acid is a type of compound that contains hydrogen and dissociates in water to produce positive hydrogen ions. The reaction for an acid HA is commonly written:
In fact, the hydrogen ion (the proton) is solvated, and the complete reaction is:
This definition of acids comes from the Arrhenius theory. Such acids tend to be corrosive substances with a sharp taste, which turn litmus red and produce colour changes with other indicators. They are referred to as protonic acids and are classified into strong acids, which are almost completely dissociated in water, (e.g. sulphuric acid and hydrochloric acid), and weak acids, which are only partially dissociated (e.g. acetic acid and hydrogen sulphide). The strength of an acid depends on the extent to which it dissociates, and is measured by its dissociation constant.
In the Lowry-Brønsted theory of acids and bases (1923), the definition was extended to one in which an acid is a proton donor (a Brønsted acid), and a base is a proton acceptor (a Brønsted base). An important feature of the Lowry-Brønsted concept is that when an acid gives up a proton, a conjugate base is formed that is capable of accepting a proton.
Similarly, every base produces its conjugate acid as a result of accepting a proton.
For example, acetate ion is the conjugate base of acetic acid, and ammonium ion is the conjugate acid of ammonia.
As the acid of a conjugate acid/base pair becomes weaker, its conjugate base becomes stronger and vice versa.
A further extension of the idea of acids and bases was made in the Lewis theory. In this, a G. N. Lewis acid is a compound or atom that can accept a pair of electrons and a Lewis base is one that can donate an electron pair. This definition encompasses "traditional" acid-base reactions, but it also includes reactions that do not involve ions, e.g.
in which NH3 is the base (donor) and BCl3 the acid (acceptor).
aspartic acid → asparaginska kiselina
Aspartic acid is an electrically charged amino acids with acidic side chains. As a group the charged amino acids are relatively abundant and are generally located on the surface of the protein. Aspartic acid and glutamic acid play important roles as general acids in enzyme active centers, as well as in maintaining the solubility and ionic character of proteins. Aspartic acid (sometimes referred to as asparate depending on pH) is non-essential in mammals, being produced from oxaloacetate by transamination.
- Abbreviations: Asp, D
- IUPAC name: 2-aminobutanedioic acid
- Molecular formula: C4H7NO4
- Molecular weight: 133.10 g/mol
Citing this page:
Generalic, Eni. "Ionska jakost otopine." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table