supercritical fluid → superkritični fluid
Supercritical fluid is any substance above its critical temperature and critical pressure (see phase diagram). It shows unique properties that are different from those of either gases or liquids under standard conditions. A supercritical fluid has both the gaseous property of being able to penetrate anything, and the liquid property of being able to dissolve materials into their components. Solublity increases with increasing density (i.e. with increasing pressure). An example of this is naphthalene which is practically insoluble in low pressure carbon dioxide. At 100 bar the solubility is 10 g/L and at 200 bar it is 50 g/L. Rapid expansion of supercritical solutions leads to precipitation of a finely divided solid.
supercritical fluid chromatography → superkritična fluidna kromatografija
Supercritical fluid chromatography (SFC) is a hybrid of gas and liquid chromatography. SFC is of importance because it permits the separation and determination of a group of compounds that are not conveniently handled by either gas or liquid chromatography. These compounds are either nonvolatile or thermally labile so that gas chromatography cannot be used and they do not contain functional groups that make possible detection by liquid chromatography. SFC has been applied to a wide variety of materials including natural prodcuts, drugs, foods, pesticides and herbicides, fossil fuels, explosives and propellants.
tear gas → suzavac
Tear gases is the common name for substances which, in low concentrations, cause pain in the eyes, flow of tears and difficulty in keeping the eyes open. Tear gases are used mainly in military exercises and in riot control, etc., but have also been used as a method of warfare. Irritating gases have been used in war since ancient times but it was not until after the Second World War that a more systematic search for effective substances was started. Among a long series of substances, three have become of greater importance than the others. These substances are chloroacetophenone (codename CN), orto-chlorobenzylidene-malononitrile (CS) and dibenz(b,f)-1,4-oxazepine (CR).
thermochemical equation → termokemijska jednadžba
Thermochemical equation is a compact equation representing a chemical reaction that describes both the stoichiometry and the energetics of the reaction. For example, the thermochemical equation
means When one mole of gaseous methane is burned in two moles of oxygen gas, one mole of carbon dioxide gas and 2 moles of steam are produced, and 2 220 kJ of heat are released.
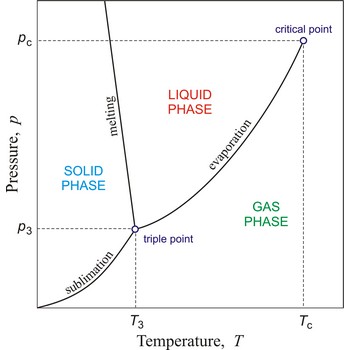
triple point → trojna točka
Triple point is the point in p,T space where the solid, liquid, and gas phases of a substance are in thermodynamic equilibrium.
U-tube manometer → U-manometar
U-tube manometer contains water or mercury in a U-shaped tube, and is usually used to measure gas pressure. One end of the U tube is exposed to the unknown pressure field (P) and the other end is connected to a reference pressure source (usually atmospheric pressure) (Pref), shown in the schematic below.
If fluid C is the atmosphere, fluid B is the liquid in the U tube (e.g. water or mercury), and fluid A is a gas, then we can assume that ρB >> ρA, ρC. The pressure contributed by the weight of gas within the U tube can therefore be neglected. The gage pressure of the gas can be approximated by,
van der Waals’ equation → van der Waalsova jednadžba
Van der Waals’ equation is an equation of state for real fluids which takes the form:
where p is pressure, Vm is molar volume, T is temperature, R is the molar gas constant, and a and b are characteristic parameters of the substance which describe the effect of attractive and repulsive intermolecular forces.
van’t Hoff equation → van Hoffova jednadžba
Van’t Hoff equation is the equation expressing the temperature dependence on the equilibrium constant K of a chemical reaction:
where ΔrH° is the standard enthalpy of reaction, R the molar gas constant, and T the temperature.
Volta, Alessandro → Volta, Alessandro
The Italian physicist Alessandro Giuseppe Antonio Anastasio Volta (1745-1827) was the inventor of the voltaic pile, the first electric battery (1800). In 1775 he invented the electrophorus, a device that, once electrically charged by having been rubbed, could transfer charge to other objects. Between 1776 and 1778, Volta discovered and isolated methane gas (CH4). The electrical unit known as the volt was named in his honor.
Citing this page:
Generalic, Eni. "Idealni plin." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table