glacial acetic acid → ledena octena kiselina
Glacial acetic acid (CH3COOH) is the pure compound, as distinguished from the usual water solutions known as acetic acid. It is a colorless liquid or crystalline substance (melting point 16.6 °C) with a pungent, vinegar odor.
Grignard reagent → Grignardov reagens
Grignard reagents are organomagnesium halides, RMgX, having a carbon- magnesium bond (or their equilibrium mixtures in solution with R2Mg + MgX2).
heat of crystallization → toplina kristalizacije
Heat of crystallization or enthalpy of crystallization is the heat evolved or absorbed when one mole of given substance crystallises from a saturated solution of the same substance.
Contat-Gockel’s valve → Contat-Gockelov ventil
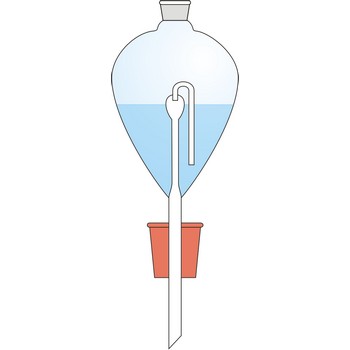
Contat-Göckel’s valve is used for maintenance of inert atmosphere in a flask. The valve is filled with a saturated solution of sodium bicarbonate (NaHCO3) so that the end of the tube is covered. Solution inside the valve keeps the flask contents away from the oxygen influence from air. If low pressure is created inside the flask (when the flask is cooled), the solution will penetrate inside it from funnel and in a reaction with acid CO2 is generated which fills up the flask.
Solution from the funnel will keep penetrating until CO2 pressure in the flask is equalised with the outer pressure.
dialysis → dijaliza
Dialysis is a method by which large molecules (such as starch or protein) and small molecules (such as glucose or amino acids) may be separated in a solution by selective diffusion through a semipermeable membrane. Through this kind of membrane dissolved particles pass and colloid dimension particles fall behind. For example, if a mixed solution of starch and glucose is placed in a closed container made of a semipermeable substance (such as cellophane), which is then immersed in a beaker of water, the smaller glucose molecules will pass trough the membrane into the water, while the starch molecules remain behind.
diffusion → difuzija
Diffusion is the spontaneous mixing of one substance with another when in contact or separated by a permeable membrane. Diffusion is a result of the random motions of their component atoms, molecules, ions, or other particles. Diffusion occurs most readily in gases, less so in liquids, and least in solids. The rate of diffusion is proportional to the concentration of the substance and increases with temperature. The theoretical principles are stated in Fick’s laws.
dissociation → disocijacija
Dissociation is the process by which a chemical combination breaks up into simpler constituents as a result of either added energy (dissociated by heat), or the effect of a solvent on a dissolved polar compound (electrolytic dissociation). It may occur in the gaseous, solid, or liquid state, or in a solution.
An example of dissociation is the reversible reaction of hydrogen iodide at high temperatures
The term dissociation is also applied to ionisation reactions of acids and bases in water. For example
which is often regarded as a straightforward dissociation into ions
Daniell cell → Daniellov članak
In 1836 the British chemist John Frederic Daniell (1790-1845) proposed an improved electric cell that supplied an even current during continuous operation. Daniell cell consisted of a glass jar containing copper and zinc electrodes, each immersed in their respective acidic sulphate solutions. The two solutions were separated by a porous clay cylinder separator. It was a galvanic cell in which the spontaneous electrodissolution of zinc and electroplating of copper provided the electrical current.
Zn(s) |
→ | Zn2+ + 2e- |
+0.763 V |
Cu2+ + 2e- |
→ | Cu(s) |
+0.337 V |
Zn(s) + Cu2+ |
→← | Zn2+ + Cu(s) |
+1.100 V |
dropping mercury electrode → kapajuća živina elektroda
Dropping mercury electrode (DME) is a working electrode arrangement for polarography in which mercury continuously drops from a reservoir through a capillary tube (internal diameter 0.03 - 0.05 mm) into the solution. The optimum interval between drops for most analyses is between 2 and 5 s. The unique advantage to the use of the DME is that the constant renewal of the electrode surface, exposed to the test solution, eliminates the effects of electrode poisoning.
immersion plating → galvaniziranje uranjanjem
Immersion plating involves depositing a metallic coating on a metal immersed in a liquid solution, without the aid of an external electric current. Also called dip plating.
Citing this page:
Generalic, Eni. "Idealna otopina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table