chlorinity → klorinitet
Originally chlorinity (symbol Cl) was defined as the weight of chlorine in grams per kilogram of seawater after the bromides and iodides had been replaced by chlorides. To make the definition independent of atomic weights, chlorinity is now defined as 0.3285233 times the weight of silver equivalent to all the halides.
The Mohr-Knudsen titration method served oceanographers for more than 60 years to determine salinity from chlorinity. This modification of the Mohr method uses special volumetric glassware calibrated directly in chlorinity units. The Mohr method uses potassium chromate (K2CrO4) as an indicator in the titration of chloride ions chloride (plus a small amount of bromide and iodide) with a silver nitrate (AgNO3) standard solution.
The other halides present are similarly precipitated.
A problem in the Mohr titration was that silver nitrate is not well suited for a primary standard. The Danish physicist Martin Knudsen (1871-1949) suggested that a standard seawater (Eau de mer Normale or Copenhagen Normal Water) be created and distributed to oceanographic laboratories throughout the world. This water was then used to standardize the silver nitrate solutions. In this way all chlorinity determinations were referred to one and the same standard which gave great internal consistency.
The relationship between chlorinity Cl and salinity S as set forth in Knudsen's tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
percolation → perkolacija
Percolation is a procedure of extraction of a material in special pipes through which a solvent is gradually released.
plastic welding → varenje plastike
Plastic welding is the fusion of two pieces (usually of the same plastic type) with a directed hot air jet and plastic welding rod. Used increasingly for the manufacture of tanks, floats and other items. Useful for repair of pipework (either temporary or permanent).
colloid → koloid
Colloids are systems in which there are two or more phases, with one (the dispersed phase) distributed in the other (the continuous phase). Moreover, at least one of the phases has small dimensions, in the range between 1 nm and 1 μm (10-9 m – 10-6 m). Dimension, rather than the nature of the material, is characteristic. In this size range, the surface area of the particle is large with respect to its volume so that unusual phenomena occur, e.g., the particles do not settle out of the suspension by gravity and are small enough to pass through filter membranes. Macromolecules (proteins and other high polymers) are at the lower limit of this range; the upper limit is usually taken to be the point at which the particles can be resolved in an optical microscope.
Colloidal particles may be gaseous, liquid, or solid, and occur in various types of suspensions:
Sols - dispersions of small solid particles in a liquid.
Emulsions - colloidal systems in which the dispersed and continuous phases are both liquids.
Gels - colloids in which both dispersed and continuous phases have a three-dimensional network throughout the material.
Aerosols - colloidal dispersions of liquid or solid particles in a gas.
Foams - dispersions of gases in liquids or solids.
colloid mill → koloidni mlin
Colloid mills are machines used to grind aggregates into very fine particles or to apply very high shearing within a fluid to produce colloid suspensions or emulsions in which the particle sizes are less than 1 micrometer. One type of colloid mill is called a disc mill, in which a mixture of a solid and liquid (or two liquids) is passed between two discs a small distance apart, which rotate very rapidly relative to each other. Applications of colloid mills occur in food processing, in paint manufacture, and in the pharmaceutical industry.
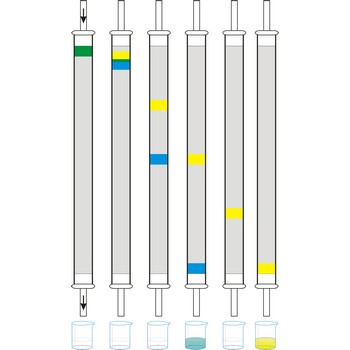
column chromatography → kromatografija u koloni
Column chromatography is generally used as a purification technique: it isolates desired compounds from a mixture. In column chromatography, the stationary phase, a solid adsorbent, is placed in a vertical column. The mobile phase, a liquid, is added to the top and flows down through the column by either gravity or external pressure. The mobile phase can be a gas or a liquid which gives rise to the two basic forms of chromatography, namely, gas chromatography (GC) and liquid chromatography (LC).
potassium fertilizer → kalijevo gnojivo
Potassium fertilizers are compounds of potassium used as an artificial manure e.g. potassium-chloride, potassium-sulphate, potassium-magnesium-sulphate, and ashes formed after wood burns out.
psychoactive drug → psihoaktivna droga
Psychoactive drugs are natural (mescaline) or synthetic substances (LSD) which take effect on central nervous system causing euphoria, and by lengthened use they also cause addiction, gradually destroying the nervous system.
reaction speed curve → krivulja brzine reakcije
Reaction speed curve is a graphic presentation of the reactant quantity change in dependence on time value.
Citing this page:
Generalic, Eni. "How many oz in a gram." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table