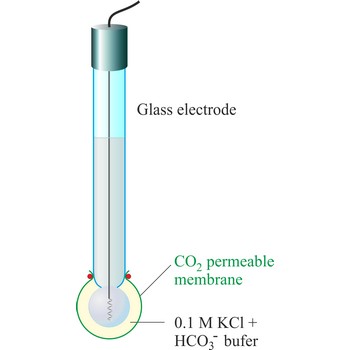
CO2 ion selective electrode → CO2 ion selektivna elektroda
The carbon dioxide ion selective electrode uses a gas-permeable membrane to separate the sample solution from the electrode internal solution. Dissolved carbon dioxide in the sample solution diffuses through the membrane until an equilibrium is reached between the partial pressure of CO2 in the sample solution and the CO2 in the internal filling solution. In any given sample the partial pressure of carbon dioxide will be proportional to the concentration of carbon dioxide. The diffusion across the membrane affects the level of hydrogen ions in the internal filling solution:
The hydrogen level of the internal filling solution is measured by the pH electrode located behind the membrane. The internal filling solution contains a high concentration of sodium bicarbonate (e.g. 0.1 mol/L NaHCO3) so that the bicarbonate level can be considered constant.
Lennard-Jones potential → Lennard-Jonesov potencijal
The Lennard-Jones potential (or 12-6 potential) is a mathematically simple model that describes the interaction between two non-bonded and uncharged atoms (known as the van der Waals interaction). It was first proposed in 1924 by British physicist Sir John Edward Lennard-Jones (1894-1954). The Lennard-Jones Potential is given by the following equation
V(r) = 4e[(sigma/r)12-(sigma/r)6)]
where V is the intermolecular potential between the two atoms or molecules, ε is the well depth and a measure of how strongly the two particles attract each other, σ is the distance at which the intermolecular potential between the two particles is zero, r is the distance of separation between centres of both particles.
Citing this page:
Generalic, Eni. "Hidrofobne interakcije." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table