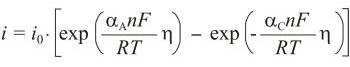critical mass → kritična masa
Critical mass is the minimum mass of a fissionable material (235U or 239Pu) that will initiate an uncontrolled chain reaction as in an atomic bomb. The critical mass of pure 239Pu is about 4.5 kg, and of 235U about 15 kg.
bomb calorimeter → kalorimetrijska bomba
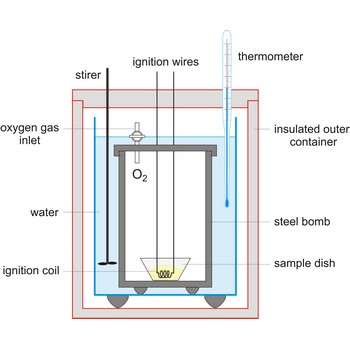
Bomb calorimeter is a type of constant-volume calorimeter used in measuring the heat of combustion of samples which can be burned in oxygen. Four essential parts are required in any bomb calorimeter:
- a bomb or vessel in which the combustible charges can be burned,
- a bucket or container for holding the bomb in a measured quantity of water, together with a stirring mechanism,
- an insulating jacket to protect the bucket from transient thermal stresses during the combustion process, and
- a thermometer or other sensor for measuring temperature changes within the bucket.
Bragg angle → Braggov kut
Bragg angle (Θ) is the angle between an incident X-ray beam and a set of crystal planes for which the secondary radiation displays maximum intensity as a result of constructive interference. British physicist Sir William Henry Bragg and his son Sir William Lawrence Bragg developed a simple relation for scattering angles, now call Bragg’s law.
which relates the angle θ between a crystal plane and the diffracted X-ray beam, the wavelength λ of the x-rays, the crystal plane spacing d, and the diffraction order n (any integer).
The diffraction experiment as presently considered is intended to provide quantitative information on the lattice constant and shape characteristics of the unit cell.
crystal → kristal
Crystal is a solid with a regular geometric shape, having a characteristic internal structure and enclosed by symmetrically arranged plane surfaces, intersecting at definite and characteristic angles. In crystals the particles (atoms, ions, or molecules) have a regular three-dimensional repeating arrangement in space. This is called the crystal structure. The crystal lattice is the arrangement of points in space at which the particles are positioned.
cyclization → ciklizacija
Cyclization is the formation of a cyclic compound from an open-chain compound.
detergent → deterdžent
Detergent is a substance added to water to improve its cleaning properties. Although water is a powerful solvent for many compounds, it will not dissolve grease and natural oils. Detergents are compounds that cause such nonpolar substances to go into solution in water. Soap is the original example, owing its action to the presence of ions formed from long-chain fatty acids ion (e.g. stearat ion, CH3(CH2)16COO-).
Butler-Volmer equation → Butler-Volmerova jednadžba
Butler-Volmer equation is an activation controlled reaction, the one for which the rate of reaction is controlled solely by the rate of the electrochemical charge transfer process, which is in turn an activation-controlled process. This gives rise to kinetics that are described by the Butler-Volmer equation:
where io is exchange current density, η is overpotential (η = E - Eo), n is number of electrons, αA is anodic transfer coefficient, and αC is cathodic transfer coefficient
calcium → kalcij
Calcium was discovered by Sir Humphry Davy (England) in 1808. The origin of the name comes from the Latin word calix meaning lime. It is fairly hard, silvery-white metal. Exposed surfaces form oxides and nitrides. Reacts with water and oxygen. Occurs only in compounds. Calcium is obtained from minerals like chalk, limestone and marble. Pure metal is produced by replacing the calcium in lime (CaCO3) with aluminium in hot, low pressure retorts. Used by many forms of life to make shells and bones. Virtually no use for the pure metal, however two of its compounds are, lime (CaO) and gypsum (CaSO4), are in great demand by a number of industries.
capacitor → kondenzator
Capacitor is a device that stores electric charges. The symbol for a capacitor in electric circuit schemes is —| |—.
Citing this page:
Generalic, Eni. "Hera chan tvb." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table