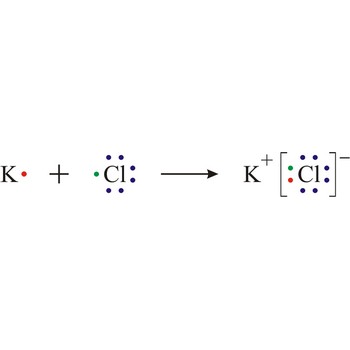helium → helij
Helium was discovered by Pierre Jules César Janssen (France) and Sir William Ramsay (Scotland) in 1868. The origin of the name comes from the Greek word helios meaning sun. It is light, odourless, colourless inert gas. Second most abundant element in the universe. Helium is found in natural gas deposits from wells in Texas, Oklahoma and Kansas. Used in balloons, deep sea diving and welding. Also used in very low temperature research.
holmium → holmij
Holmium was discovered by Per Theodore Cleve (Sweden) in 1879. The origin of the name comes from the Greek word Holmia meaning Stockholm. It is fairly soft, malleable, lustrous, silvery metal. Reacts slowly with oxygen and water. Dissolves in acids. Can react violently with air or halogens. Holmium occurs in gadolinite. Most often from monazite sand. It has very few practical applications; however, it has some unusual magnetic properties that offer some hope for future applications.
hydration → hidratacija
Hydration is addition of water or the elements of water (i.e. H and OH) to a molecular entity. The term is also used in a more restricted sense for the process:
hydrogen → vodik
Hydrogen was discovered by Sir Henry Cavendish (England) in 1766. The origin of the name comes from the Greek words hydro and genes meaning water and generate. It is colourless, odourless gas, burns and forms explosive mixtures in air. Reacts violently with oxidants. Hydrogen is the most abundant element in the universe. Commercial quantities of hydrogen are produced by reacting superheated steam with methane or carbon. In lab work from reaction of metals with acid solutions or electrolysis. Most hydrogen is used in the production of ammonia and in metal refining. Also used as fuel in rockets. Its two heavier isotopes (deuterium and tritium) used respectively for nuclear fusion.
iron → željezo
Iron has been known since ancient times. The origin of the name comes from the Latin word ferrum meaning iron. It is malleable, ductile, silvery-white metal. Exposed surfaces form red-brown oxides. Forms very strong alloys (steel). Ferromagnetic. Metal dust flammable. Fourth most abundant element in the earth’s crust. Iron is obtained from iron ores. Pure metal produced in blast furnaces by layering limestone, coke and iron ore and forcing hot gasses into the bottom. This heats the coke red hot and the iron is reduced from its oxides and liquefied where it flows to the bottom. Iron is the most common metal in human society. More than 90 % of all metal refined in the world is iron. Used in steel and other alloys. It is the chief constituent of hemoglobin which carries oxygen in blood vessels. Its oxides are used in magnetic tapes and disks.
Kirchoff, Gustav → Kirchoff, Gustav
Gustav Kirchoff (1824-1887) was a German physicist who, with the chemist Robert Bunsen (1811-1899), laid the foundations of spectral analysis. He realized that the Fraunhofer lines in the Sun's spectrum were due to light from the photosphere being absorbed at those specific wavelengths by elements in the solar atmosphere. He also found that incandescent solids, liquids, and compressed gases emit a continuous spectrum. Use of the Bunsen burner in conjunction with a glass prism led to the development of the spectroscope in collaboration with the Bunsen and to the spectroscopic discovery of the elements rubidium (1860) and cesium (1861).
lanthanides → lantanoidi
Lanthanides (lanthanons, lanthanoids or rare-earth elements) are a series of fourteen elements in the periodic table, generally considered to range in proton number from cerium to lutetium inclusive. It was convenient to divide these elements into the cerium group or light earth: cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu); and the yttrium group or heavy earths: gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb) i lutetium (Lu). The position of lanthanum is somewhat equivocal and, although not itself a lanthanide, it is often included with them for comparative purpose. The lanthanides are sometimes simply called the rare earths. Apart from unstable Pm, the lanthanides are actually not rare. Cerium is the 26. most abundant of all elements, 5 times as abundant as Pb. All are silvery very reactive metals.
Lewis structure → Lewisova struktura
Lewis structure is the representation of the electron arrangement in atoms, ions, or molecules by showing the valence electrons as dots placed around the symbols for the elements.
lithium → litij
Lithium was discovered by Johan August Arfvedson (Sweden) in 1817. The origin of the name comes from the Greek word lithos meaning stone, apparently because it was discovered from a mineral source whereas the other two elements, sodium and potassium, were discovered from plant sources. It is soft silvery-white metal. Lightest of metals. Reacts slowly with water and oxygen. Flammable. Can ignite in air. Reacts with water to give off a flammable gas. Lithium is obtained by passing electric charge through melted lithium chloride and from the silicate mineral called spodumene [LiAl(Si2O6)]. Used in batteries. Also for certain kinds of glass and ceramics. Some is used in lubricants.
metal → metal
Metals are materials in which the highest occupied energy band (conduction band) is only partially filled with electrons.
Their physical properties generally include:
- They are good conductors of heat and electricity. The electrical conductivity of metals generally decreases with temperature.
- They are malleable and ductile in their solid state.
- They show metallic lustre.
- They are opaque.
- They have high density.
- They are solids (except mercury)
- They have a crystal structure in which each atom is surrounded by eight to twelve near neighbours
Their chemical properties generally are:
- They have one to four valence electrons.
- They have low ionisation potentials; they readily lose electrons.
- They are good reducing agents.
- They have hydroxides which are bases or amphoteric.
- They are electropositive.
Metallic characteristics of the elements decrease and non-metallic characteristics increase with the increase of valence electrons. Also metallic characteristics increase with the number of electron shells. Therefore, there is no sharp dividing line between the metals and non-metals.
Of the 114 elements now known, only 17 show primarily non-metallic characteristics, 7 others are metalloids, and 89 may be classed as metals.
Citing this page:
Generalic, Eni. "Halogeni elementi." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table