accelerator → akcelerator
Accelerator is a device (machine) used for acceleration of charged particles (protons, deuterons, α-particles). Particles are accelerated under the influence of an electric field and with the help of a magnetic field are kept inside a certain space. When the particles reach enough acceleration (that is sufficient energy), they are directed on a target we wish to bomb. Best known types cyclotron, synchrotron, betatron.
Accelerator is a substance that increases the rate of chemical reaction, i.e. a catalyst.
accumulator → akumulator
Accumulator (secondary cell, storage battery) is a type of voltaic cell or battery that can be recharged by passing current through it from an external D.C. supply. The charging current reverses the chemical reactions in the cell. The common types are the lead-acid accumulator and the nickel-cadmium cell.
activated complex → aktivirani kompleks
Activated complex is an intermediate structure formed in the conversion of reactants to products. The activated complex is the structure at the maximum energy point along the reaction path; the activation energy is the difference between the energies of the activated complex and the reactants.
biogas → bioplin
Biogas is a mixture of methane and carbon dioxide resulting from the anaerobic decomposition of such waste materials as domestic, industrial, and agricultural sewage. Methanogenic bacteria carry out the decomposition; these obligate anaerobes produce methane, the main component of biogas, which can be collected and used as an energy source for domestic processes, such as heating, cooking, and lighting.
chemiluminescence → kemiluminiscencija
Chemiluminescence is energy release in form of electromagnetic radiation during a chemical reaction.
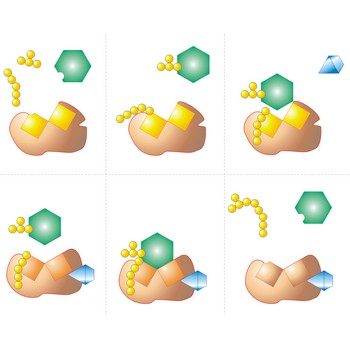
active site → aktivno mjesto
Active site is a pocket or crevice on an enzyme molecule that fits reactant molecules like a hand in a glove. The active site lowers the activation energy for reaction
adenosine triphosphate → adenozin trifosfat
Adenosine triphosphate (ATP) is nucleotide that is of fundamental importance as a carrier of chemical energy in all living organisms. It consists of adenin linked to D-ribose).
Arrhenius equation → Arheniusova jednadžba
In 1889, Svante Arrhenius explained the variation of rate constants with temperature for several elementary reactions using the relationship
where the rate constant k is the total frequency of collisions between reaction molecules A times the fraction of collisions exp(-Ea/RT) that have an energy that exceeds a threshold activation energy Ea at a temperature of T (in kelvin). R is the universal gas constant.
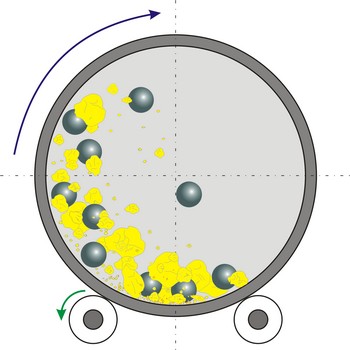
ball mill → kuglični mlin
Ball mill is a grinder for reducing hard materials to powder. The grinding is carried out by the pounding and rolling of a charge of steel or ceramic balls carried within the cylinder. The cylinder rotates at a relatively slow speed, allowing the balls to cascade through the mill base, thus grinding or dispersing the materials.
Type of ball mills, centrifugal and planetary mills, are devices used to rapidly grind materials to colloidal fineness (approximately 1 μm and below) by developing high grinding energy via centrifugal and/or planetary action.
Citing this page:
Generalic, Eni. "Energija ionizacije." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table