chemical element → kemijski element
Chemical element is a type of matter of which elementary matter is composed. Chemical element is composed of atoms with the same core charge.
electron → elektron
The electron is an elementary particle with a negative electric charge of (1.602 189 2±0.000 004 6)×10-19 C and a mass of 1/1837 that of a proton, equivalent to (9.109 534±0.000 047)×10-31 kg.
In 1897 the British physicist Joseph John (J.J.) Thomson (1856-1940) discovered the electron in a series of experiments designed to study the nature of electric discharge in a high-vacuum cathode-ray tube. Thomson interpreted the deflection of the rays by electrically charged plates and magnets as evidence of bodies much smaller than atoms that he calculated as having a very large value for the charge to mass ratio. Later he estimated the value of the charge itself.
Electrons are arranged in from one to seven shells around the nucleus; the maximum number of electrons in each shell is strictly limited by the laws of physics (2n2). The outer shells are not always filled: sodium has two electrons in the first shell (2×12 = 2), eight in the second (2×22 = 8), and only one in the third (2×32 = 18). A single electron in the outer shell may be attracted into an incomplete shell of another element, leaving the original atom with a net positive charge. Valence electrons are those that can be captured by or shared with another atom.
Electrons can be removed from the atoms by heat, light, electric energy, or bombardment with high-energy particles. Decaying radioactive nuclei spontaneously emit free electrons, called β particles.
halogens → halogeni elementi
Halogens are the elements fluorine (F) chlorine (Cl), bromine (Br), iodine (I), and astatine (At). They are non-metals, and make up part of the 17 group in the periodic table. Compounds of these elements are called halogenides or halides.
The halogens all have a strong unpleasant odour and will burn flesh. They do not dissolve well in water. The five elements are strongly electronegative. They are oxidising agents, with fluorine being the strongest and astatine being the weakest. They react with most metals and many non-metals.
Halogens form molecules which consist of atoms covalently bonded. With increasing atomic weight there is a gradation in physical properties. For example: Fluorine is a pale green gas of low density. Chlorine is a greenish-yellow gas 1.892 times as dense as fluorine. Bromine is a deep reddish-brown liquid which is three times as dense as water. Iodine is a grayish-black crystalline solid with a metallic appearance. And astatine is a solid with properties which indicate that it is somewhat metallic in character.
mole → mol
Mole (mol) is the SI base unit of amount of substance.
The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kg of carbon 12.
When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles. In this definition, it is understood that the carbon 12 atoms are unbound, at rest and in their ground state.
unit cell → jedinična ćelija
Unit cell is the smallest fragment of the structure of a solid that by repetition can generate the entire structure.
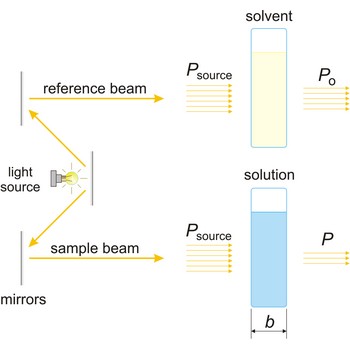
absorbance → apsorbancija
Absorbance (A) is a logarithm of the ratio of incident radiant power (Po) to transmitted radiant power (P) through a sample (excluding the effects on cell walls).
The absorption of light by a substance in a solution can be described mathematically by the Beer-Lambert law
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
absorbed dose → apsorbirana doza
For any ionising radiation, absorbed dose (D) is the mean energy imparted to an element of irradiated matter divided by the mass of that element.
absorption → apsorpcija
Absorption is a phenomenon that occurs when matter crosses from one phase to another passing through the border surface and in the other phase more or less monotonously distributes itself in a concentration higher than the one within the first phase.
abundance of elements → rasprostranjenost elemenata
Elements in nature are mostly found in different compounds and, rarely, in the free (elementary) state. In Earth’s crust the most abundant of all elements is oxygen (with 49.5 %), then silicon (25 %), aluminium (7.5 %), iron (4.7 %), calcium (3.4 %), sodium (2.6 %), potassium (2.4 %), magnesium (1.9 %) and hydrogen (1.9 %). These nine elements make up almost 99 % of the Earth’s composition.
Citing this page:
Generalic, Eni. "Elementarna tvar." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table