cathode → katoda
Cathode is a negative electrode of an electrolytic cell to which positively charged ions (cations) migrate when a current is passed as in electroplating baths.
In a primary or secondary cell (battery or accumulator) the cathode is the electrode that spontaneously becomes negative during discharge, and form which therefore electrons emerge.
In vacuum electronic devices electrons are emitted by the cathode and flow to the anode.
cathode ray → katodna zraka
Cathode ray is a negatively charged beam that emanates from the cathode of a discharge tube. Cathode rays are streams of electrons.
cathodic protection → katodna zaštita
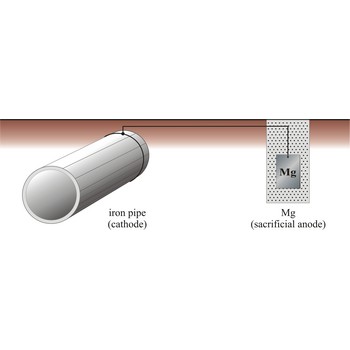
Cathodic protection is a process in which a structural metal, such as iron, is protected from corrosion by connecting it to a metal that has a more negative reduction half-cell potential, which now corrodes instead of iron. There are two major variations of the cathodic method of corrosion protection. The first is called the impressed current method, and the other is called the sacrificial anode method.
elementary particle → elementarna čestica
Elementary particle is one of fundamental particles of which matter is composed, such as the electron, proton or neutron.
equivalent weight → ekvivalentna masa
Equivalent weight of a substance participating in a neutralization reaction is that mass of substance (molecule, ion, or paired ion) that either reacts with or supplies 1 mol of hydrogen ions in that reaction.
Equivalent weight of a substance participating in an oxidation/reduction reaction is that weight which directly or indirectly produces or consumes 1 mol of electrons.
farad → farad
Farad (F) is the SI derived unit of electric capacitance. The farad is the capacitance of an electric capacitor between the two plates of which there appears a difference of electric potential of one volt when it is charged by a quantity of electricity equal to one coulomb (F = C/V). The unit was named after the British scientist M. Faraday (1791-1867).
cell potential → potencijal članka
Cell potential (E) is difference between anode and cathode potential. If the cell potential is positive, then the reaction is spontaneous.
chlorine → klor
Chlorine was discovered by Carl William Scheele (Sweden) in 1774. The origin of the name comes from the Greek word chloros meaning pale green. It is greenish-yellow, disagreeable gas with irritating odour. Gas is toxic and severe irritant by contact or inhalation. Never found in free form in nature. Commercial quantities of chlorine are produced by electrolysis of aqueous sodium chloride (NaCl) from seawater or brine from salt mines. Used in water purification, bleaches, acids and many, many other compounds such as chlorofluorocarbons (CFC).
coenzyme q → koenzim q
Coenzyme Q (CoQ) or ubiquinone is any of a group of related quinone-derived compounds that serve as electron carriers in the electron transport chain reactions of cellular respiration. There are some differences in the length of the isoprene unit (in bracket on left) side chain in various species. All the natural forms of CoQ are insoluble in water, but soluble in membrane lipids.
colloid ion → koloidni ion
Colloid ions emerge when colloid particles adsorb certain type of ion from solution and thus become charged with the same charge. The charge can also originate form a chemical reaction of colloid particle’s surface. Colloid ions formed by absorption of silver chloride particle can be show as follows:
Adsorbed layer is monomolecular (one molecule thick) and which type of ion will be formed depends upon which ions are present in a greater number in the solution in. Because of this colloid particles are charged with the same charge, mutual repelling occurs, and the colloid solution becomes stable. Colloid charge can be determined by electrophoresis.
Citing this page:
Generalic, Eni. "Elektron." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table