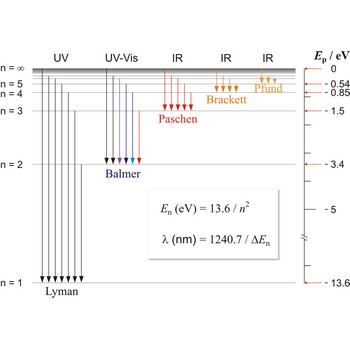
Balmer series → Balmerova serija
Balmer series, Balmer lines is a series of lines in the emission spectrum of hydrogen that involve transitions to the n=2 state from states with n>2.
Bohr atom → Bohrov atom
Bohr atom is a model of the atom that explains emission and absorption of radiation as transitions between stationary electronic states in which the electron orbits the nucleus at a definite distance. The Bohr model violates the Heisenberg uncertainty principle since it postulates definite paths and moment for electrons as they move around the nucleus. Modern theories usually use atomic orbitals to describe the behaviour of electrons in atoms.
chemiluminescence → kemiluminiscencija
Chemiluminescence is energy release in form of electromagnetic radiation during a chemical reaction.
Gaussian system of units → Gaussov sustav jedinica
Gaussian system of units is a hybrid system used in electromagnetic theory, which combines features of both the electrostatic cgs subsystem (esu) and electromagnetic cgs subsystem (emu). With three base units, it uses em units in magnetism and es units in electrostatics. This involves using the constant c (the velocity of light in vacuum) to interrelate these sets of units.
geometrical optics → geometrijska optika
In most cases light can be described as an electromagnetic wave. Geometrical optics is an approximation in which the waves can be represented as straight-line rays. This approximation is valid if the light waves do not meet obstacles comparable in size to the wavelength of radiation.
gamma radiation → gama-zračenje
Gamma radiation is electromagnetic radiation of extremely short wavelength. Gamma radiation ranges in energy from about 10-15 J to 10-10 J (10 keV to 10 MeV) (wavelength less than about 1 pm). Gamma rays are emitted by excited atomic nuclei during the process of passing to a lower excitation state.
Gamma rays are extremely penetrating and are absorbed by dense materials like lead and uranium. Exposure to gamma radiation may be lethal.
luminescence → luminiscencija
Luminescence (from Latin lumen, light) is the emission of electromagnetic radiation (UV, visible or IR) from atoms or molecules as a result of the transition of an electronically excited state to a lower energy state, usually the ground state. Luminescence can be divided into categories by duration (fluorescence or phosphorescence) or by the mechanism that creates the light (radioluminescence, electroluminescence, photoluminescence, thermoluminescence, triboluminescence, chemiluminescence, bioluminescence). The prefix identifies the energy source responsible for generating or releasing the light.
Phosphorescence is emission of light from a substance exposed to radiation and persisting as an afterglow after the source of excitation has been removed. Fluorescence, on the other hand, is an almost instantaneous effect, ending within about 10-8 second after excitation.
microwave radiation → mikrovalna radijacija
Microwave radiation is a electromagnetic radiation with wavelength between 3 mm and 30 cm.
Citing this page:
Generalic, Eni. "Elektromagnetski spektar." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table