positive pole → pozitivni pol
Positive pole is that half-cell in the electrochemical cell which has the most positive electrode potential.
galvanic celll → galvanski članak
Galvanic cell (voltaic cell) is a simple device with which chemical energy is converted into electrical energy. Galvanic cells consist of two separate compartments called half cells containing electrolyte solutions and electrodes that can be connected in a circuit. Two dissimilar metals (e.g., copper and zinc) are immersed in an electrolyte. If the metals are connected by an external circuit, one metal is reduced (i.e., gains electrons) while the other metal is oxidized (i.e., loses electrons).
In the example above, copper is reduced and zinc is oxidized. The difference in the oxidation potentials of the two metals provides the electric power of the cell.
A voltaic cell can be diagrammed using some simple symbols. In the diagram the electrodes are on the outer side of the diagram and a vertical line (|) is used to separate the electrode from the electrolyte solution found in the compartment. A double vertical line (||) is used to separate the cell compartments and is symbolic of the salt bridge. Usually in a diagram the species oxidized is written to the left of the double slash. Here is an example of the Daniell cell:
The names refer to the 18th-century Italian scientists Alessandro Volta (1745-1827) and Luigi Galvani (1737-1798).
lithium → litij
Lithium was discovered by Johan August Arfvedson (Sweden) in 1817. The origin of the name comes from the Greek word lithos meaning stone, apparently because it was discovered from a mineral source whereas the other two elements, sodium and potassium, were discovered from plant sources. It is soft silvery-white metal. Lightest of metals. Reacts slowly with water and oxygen. Flammable. Can ignite in air. Reacts with water to give off a flammable gas. Lithium is obtained by passing electric charge through melted lithium chloride and from the silicate mineral called spodumene [LiAl(Si2O6)]. Used in batteries. Also for certain kinds of glass and ceramics. Some is used in lubricants.
salt bridge → solni most
Salt bridge is a permeable material soaked in a salt solution that allows ions to be transferred from one container to another. The salt solution remains unchanged during this transfer.
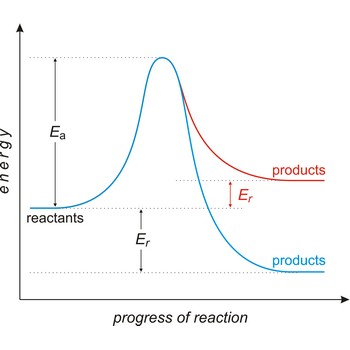
activation energy → energija aktivacije
Activation energy (Ea) is the energy that must be added to a system in order for a process to occur, even though the process may already be thermodynamically possible. In chemical kinetics, the activation energy is the height of the potential barrier separating the products and reactants. It determines the temperature dependence on the reaction rate.
battery → baterija
Battery a device that converts chemical energy to electrical energy. The process underlying the operation of a battery involves a chemical reaction in which electrons are transferred from one chemical species to another. This process is carried out in two half-reactions, one that involves the loss of electrons and one that involves their gain. The battery is an electrochemical cell divided in two half-cells, and reaction proceeds when these are connected together by an electrically conducting pathway. The passage of electrons from one half-cell to the other corresponds to an electric current. Each half-cell contains an electrode in contact with the reacting species. The electrode which passes electrons into the circuit when battery discharges is called anode and is negative terminal. The electrode which receives electrons is called cathode, and is the battery’s positive terminal. The electrical circuit is completed by an electrolyte, an electrically conducting substance placed between the two electrodes which carriers a flow of charge between them. In wet cells, the electrolyte is a liquid containing dissolved ions, whose motion generates an electrical current; in dry cells the electrolyte is basely solid, for example, a solid with mobile ions or porous solid saturated with an ionic solution.
coulometry → kulometrija
Coulometry is a quantitative electrochemical analytical method which is based on measuring the quantity of electricity that has passed and on Faraday’s laws.
calomel electrode → kalomel elektroda
Calomel electrode is a type of half cell in which the electrode is mercury coated with calomel (Hg2Cl2) and the electrolyte is a solution of potassium chloride and saturated calomel. In the calomel half cell the overall reaction is
Table: Dependence of potential of calomel electrode upon temperature and concentration of KCl according to standard hydrogen electrode
| Potential vs. SHE / V | |||
|---|---|---|---|
| t / °C | 0.1 mol dm-3 | 3.5 mol dm-3 | sat. solution |
| 15 | 0.3362 | 0.254 | 0.2511 |
| 20 | 0.3359 | 0.252 | 0.2479 |
| 25 | 0.3356 | 0.250 | 0.2444 |
| 30 | 0.3351 | 0.248 | 0.2411 |
| 35 | 0.3344 | 0.246 | 0.2376 |
electroanalytical chemistry → elektroanalitička kemija
Electroanalytical chemistry chemistry is the application of electrochemical cells and electrochemical techniques for chemical analysis. The analyte is dissolved in the electrolyte of the cell, and one can perform either qualitative analysis (determination of the type of constituents present) or quantitative analysis (determination of the amount of a given constituent).
electromotive force → elektromotorna sila
Electromotive force (e.m.f. or EMF) is the difference in electric potential that exists between two dissimilar electrodes immersed in the same electrolyte or otherwise connected by ionic conductors.
Citing this page:
Generalic, Eni. "Elektrokemijski potencijal." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table