lead-acid battery → olovni akumulator
Lead-acid battery is a electrical storage device that uses a reversible chemical reaction to store energy. It was invented in 1859 by French physicist Gaston Planté. Lead-acid batteries are composed of a lead(IV) oxide cathode, a sponge metallic lead anode and a sulphuric acid solution electrolyte.
In charging, the electrical energy supplied to the battery is changed to chemical energy and stored. The chemical reaction during recharge is normally written:
In discharging, the chemical energy stored in the battery is changed to electrical energy. During discharge, lead sulfate (PbSO4) is formed on both the positive and negative plates. The chemical reaction during discharge is normally written:
Lead acid batteries are low cost, robust, tolerant to abuse, tried and tested. For higher power applications with intermittent loads however, they are generally too big and heavy and they suffer from a shorter cycle life.
mercury → živa
Mercury has been known since ancient times. The origin of the name comes from the Latin word hydrargyrum meaning liquid silver. It is heavy, silver-white metal, liquid at ordinary temperatures. Stable in air and water. Unreactive with alkalis and most acids. Gives off poisonous vapour. Chronic cumulative effects. Mercury only rarely occurs free in nature. The chief ore is cinnabar or mercury sulfide (HgS). Used in thermometers, barometers and batteries. Also used in electrical switches and mercury-vapour lighting products.
metal → metal
Metals are materials in which the highest occupied energy band (conduction band) is only partially filled with electrons.
Their physical properties generally include:
- They are good conductors of heat and electricity. The electrical conductivity of metals generally decreases with temperature.
- They are malleable and ductile in their solid state.
- They show metallic lustre.
- They are opaque.
- They have high density.
- They are solids (except mercury)
- They have a crystal structure in which each atom is surrounded by eight to twelve near neighbours
Their chemical properties generally are:
- They have one to four valence electrons.
- They have low ionisation potentials; they readily lose electrons.
- They are good reducing agents.
- They have hydroxides which are bases or amphoteric.
- They are electropositive.
Metallic characteristics of the elements decrease and non-metallic characteristics increase with the increase of valence electrons. Also metallic characteristics increase with the number of electron shells. Therefore, there is no sharp dividing line between the metals and non-metals.
Of the 114 elements now known, only 17 show primarily non-metallic characteristics, 7 others are metalloids, and 89 may be classed as metals.
non-metal → nemetal
Non-metals are defined as elements that are not metals.
Their physical properties generally include:
- They are poor conductors.
- They are brittle, not ductile in their solid state.
- They show no metallic lustre.
- They may be transparent or translucent.
- They have low density.
- They form molecules which consists of atoms covalently bonded; the noble gases are monoatomic.
Their chemical properties are generally:
- They usually have four to eight valence electrons.
- They have high electron affinities (except the noble gases)
- They are good oxidising agents (except the noble gases)
- They have hydroxides which are acidic (except the noble gases)
- They are electronegative.
nuclear magnetic resonance → nuklearna magnetska rezonancija
Nuclear magnetic resonance (NMR) is a type of radio-frequency spectroscopy based on the magnetic field generated by the spinning of electrically charged atomic nuclei. This nuclear magnetic field is caused to interact with a very large (1 T - 5 T) magnetic field of the instrument magnet. NMR techniques have been applied to studies of electron densities and chemical bonding and have become a fundamental research tool for structure determinations in organic chemistry.
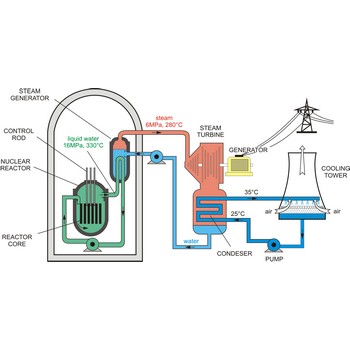
nuclear reactor → nuklearni reaktor
Nuclear reactor is an assembly of fissionable material (uranium-235 or plutonium-239) designed to produce a sustained and controllable chain reaction for the generation of electric power.
The essential components of a nuclear reactor are:
- The core, metal rods containing enough fissionable material to maintain a chain reaction at the necessary power level (as much as 50 t of uranium may be required).
- A source of neutrons to initiate the reaction (such as a mixture of polonium and beryllium)
- A moderator to reduce the energy of fast neutrons for more efficient fission (material such as graphite, beryllium, heavy water, and light water are used)
- A coolant to remove the fission-generated heat (water, sodium, helium, and nitrogen may be used)
- A control system such as rods of boron or cadmium that have high capture cross sections (to absorb neutrons)
- Adequate shielding, remote-control equipment, and appropriate instrumentation are essential for personnel safety and efficient operation.
palladium → paladij
Palladium was discovered by William Hyde Wollaston (England) in 1803. Named after the asteroid Pallas which was discovered at about the same time and from the Greek name Pallas, goddess of wisdom. It is soft, malleable, ductile, silvery-white metal. Resists corrosion; dissolves in oxidizing acids. Absorbs hydrogen. Metal dust is combustible. Palladium is obtained with platinum, nickel, copper and mercury ores. Used as a substitute for silver in dental items and jewellery. The pure metal is used as the delicate mainsprings in analog wristwatches. Also used in surgical instruments and as catalyst.
phosphorus → fosfor
Phosphorus was discovered by Hennig Brandt (Germany) in 1669. The origin of the name comes from the Greek word phosphoros meaning bringer of light. White phosphorus is white to yellow soft, waxy phosphorescent solid with acrid fumes. Toxic by inhalation, ingestion, or skin contact. Red phosphorus is powdery, non-flammable and non-toxic. Phosphorus is found most often in phosphate rock. Pure form is obtained by heating a mixture of phosphate rock, coke and silica to about 1450 °C. Used in the production of fertilizers and detergents. Some is used in fireworks, safety matches and incendiary weapons. Phosphorus is also important in the production of steels, phosphor bronze and many other products.
plastic → plastika
Plastic is a material that can be shaped by the application of heat or pressure. Most are based on synthetic polymers although some are the product of natural substances (such as cellulose derivatives, but excluding the rubbers.). They are usually light and permanent solids, being also heat and electric isolators. If the materials soften again when reheated, they are said to be thermoplastic. If, after fashioning, they resist further applications of heat, they are said to be thermoset.
Citing this page:
Generalic, Eni. "Električno polje." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table