manganese → mangan
Manganese was discovered by Johann Gahn (Sweden) in 1774. The origin of the name comes from the Latin word magnes meaning magnet, or magnesia nigri meaning black magnesia (MnO2). It is hard, brittle, grey-white metal with a pinkish tinge. Impure forms are reactive. Rusts like iron in moist air. Manganese is most abundant ores are pyrolusite (MnO2), psilomelane [(Ba,H2O)2Mn5O10] and rhodochrosite (MnCO3). Pure metal produced by mixing MnO2 with powered Al and ignited in a furnace. Used in steel, batteries and ceramics. The steel in railroad tracks can contain as much as 1.2 % manganese. It is crucial to the effectiveness of vitamin B1.
lead-acid battery → olovni akumulator
Lead-acid battery is a electrical storage device that uses a reversible chemical reaction to store energy. It was invented in 1859 by French physicist Gaston Planté. Lead-acid batteries are composed of a lead(IV) oxide cathode, a sponge metallic lead anode and a sulphuric acid solution electrolyte.
In charging, the electrical energy supplied to the battery is changed to chemical energy and stored. The chemical reaction during recharge is normally written:
In discharging, the chemical energy stored in the battery is changed to electrical energy. During discharge, lead sulfate (PbSO4) is formed on both the positive and negative plates. The chemical reaction during discharge is normally written:
Lead acid batteries are low cost, robust, tolerant to abuse, tried and tested. For higher power applications with intermittent loads however, they are generally too big and heavy and they suffer from a shorter cycle life.
mass spectrometry → masena spectrometrija
Mass spectrometry is an analytical technique in which ions are separated according to the mass/charge (m/e) ratio and detected by a suitable detector.
In a mass spectrometer a sample is ionised and the positive ions produced are accelerated into a high-vacuum region containing electric and magnetic fields. These fields deflect and focus the ions onto a detector. A mass spectrum is thus obtained, consisting of a series of peaks of variable intensity to which m/e values can be assigned. Different molecules can be identified by their characteristic pattern of lines.
mercury → živa
Mercury has been known since ancient times. The origin of the name comes from the Latin word hydrargyrum meaning liquid silver. It is heavy, silver-white metal, liquid at ordinary temperatures. Stable in air and water. Unreactive with alkalis and most acids. Gives off poisonous vapour. Chronic cumulative effects. Mercury only rarely occurs free in nature. The chief ore is cinnabar or mercury sulfide (HgS). Used in thermometers, barometers and batteries. Also used in electrical switches and mercury-vapour lighting products.
metal → metal
Metals are materials in which the highest occupied energy band (conduction band) is only partially filled with electrons.
Their physical properties generally include:
- They are good conductors of heat and electricity. The electrical conductivity of metals generally decreases with temperature.
- They are malleable and ductile in their solid state.
- They show metallic lustre.
- They are opaque.
- They have high density.
- They are solids (except mercury)
- They have a crystal structure in which each atom is surrounded by eight to twelve near neighbours
Their chemical properties generally are:
- They have one to four valence electrons.
- They have low ionisation potentials; they readily lose electrons.
- They are good reducing agents.
- They have hydroxides which are bases or amphoteric.
- They are electropositive.
Metallic characteristics of the elements decrease and non-metallic characteristics increase with the increase of valence electrons. Also metallic characteristics increase with the number of electron shells. Therefore, there is no sharp dividing line between the metals and non-metals.
Of the 114 elements now known, only 17 show primarily non-metallic characteristics, 7 others are metalloids, and 89 may be classed as metals.
metallic glass → metalno staklo
Certain alloys can solidify by extremely rapid cooling out of melt without formation of a crystal lattice, that is in the amorphous form - such, amorphous alloys are so called metallic glasses. The alloy of zirconium, beryllium, titanium, copper, and nickel is one of the first metallic glasses that can be made in bulk and formed into strong, hard, useful objects.
Unlike pure metals and most metal alloys, metallic glasses have no regular crystalline structure. This lack of long range order or microstructure is related to such desirable features as strength and low damping which is one reason why the premier use for zirconium-based metallic glass is in the manufacture of expensive golf club heads. Metallic glasses can be quite strong yet highly elastic, and they can also be quite tough (resistant to fracture). Even more interesting are the thermal properties; for instance, just like an oxide glass, there is a temperature (called the glass transition temperature) above which a metallic glass becomes quite soft and flows easily. This means that there are lots of opportunities for easily forming metallic glasses into complex shapes.
Moh’s scale → Mohsova skala
Mohs’ scale of mineral hardness characterises the scratch resistance of various minerals through the ability of a harder material to scratch a softer. It was created by the German mineralogist Friedrich Mohs (1773-1839). Mohs based the scale on the ten readily available minerals.
| Hardness | Mineral |
|---|---|
| 1 | talc (Mg3Si4O10(OH)2) |
| 2 | gypsum (CaSO4·2H2O) |
| 3 | calcite (CaCO3) |
| 4 | fluorite (CaF2) |
| 5 | apatite (Ca5(PO4)3(OH-,Cl-,F-)) |
| 6 | orthoclase feldspar (KAlSi3O8) |
| 7 | quartz (SiO2) |
| 8 | topaz (Al2SiO4(OH-,F-)2) |
| 9 | corundum (Al2O2) |
| 10 | diamond (C) |
non-metal → nemetal
Non-metals are defined as elements that are not metals.
Their physical properties generally include:
- They are poor conductors.
- They are brittle, not ductile in their solid state.
- They show no metallic lustre.
- They may be transparent or translucent.
- They have low density.
- They form molecules which consists of atoms covalently bonded; the noble gases are monoatomic.
Their chemical properties are generally:
- They usually have four to eight valence electrons.
- They have high electron affinities (except the noble gases)
- They are good oxidising agents (except the noble gases)
- They have hydroxides which are acidic (except the noble gases)
- They are electronegative.
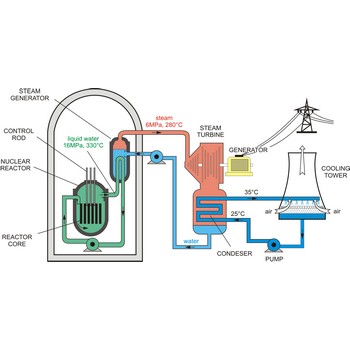
nuclear reactor → nuklearni reaktor
Nuclear reactor is an assembly of fissionable material (uranium-235 or plutonium-239) designed to produce a sustained and controllable chain reaction for the generation of electric power.
The essential components of a nuclear reactor are:
- The core, metal rods containing enough fissionable material to maintain a chain reaction at the necessary power level (as much as 50 t of uranium may be required).
- A source of neutrons to initiate the reaction (such as a mixture of polonium and beryllium)
- A moderator to reduce the energy of fast neutrons for more efficient fission (material such as graphite, beryllium, heavy water, and light water are used)
- A coolant to remove the fission-generated heat (water, sodium, helium, and nitrogen may be used)
- A control system such as rods of boron or cadmium that have high capture cross sections (to absorb neutrons)
- Adequate shielding, remote-control equipment, and appropriate instrumentation are essential for personnel safety and efficient operation.
Citing this page:
Generalic, Eni. "Električna otpornost." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table