cathode → katoda
Cathode is a negative electrode of an electrolytic cell to which positively charged ions (cations) migrate when a current is passed as in electroplating baths.
In a primary or secondary cell (battery or accumulator) the cathode is the electrode that spontaneously becomes negative during discharge, and form which therefore electrons emerge.
In vacuum electronic devices electrons are emitted by the cathode and flow to the anode.
cathode ray → katodna zraka
Cathode ray is a negatively charged beam that emanates from the cathode of a discharge tube. Cathode rays are streams of electrons.
irreversible galvanic cell → nepovrativi galvanski članak
Irreversible galvanic cell is a chemical source of direct current, in which reactions that take place on the electrodes are irreversible.
metalloid → polumetal
Metalloid (semimetal) is any of a class of chemical elements intermediate in properties between metals and nonmetals. The classification is not clear cut, but typical metalloids are boron (B), silicon (Si), germanium (Ge), arsenic (As), and tellurium (Te). They are electrical semiconductors and their oxides are amphoteric.
nonelectrolyte → neelektrolit
Nonelectrolytes are substances which in solutions do not dissociate into ions and they do not conduct electric current.
ohm → om
Ohm (Ω) is the SI derived unit of electric resistance. The ohm is the electric resistance between two points of a conductor when a constant difference of potential of one volt, applied between these two points, produces in this conductor a current of one ampere, this conductor not being the source of electromotive force (Ω = V/A). The unit was named after the German physicist Georg Simon Ohm (1789-1854).
overpotential → prenapon
Overpotential (η) is a potential that must be applied in an electrolytic cell in addition to the theoretical potential required to liberate a given substance at an electrode. The value depends on the electrode material and on the current density.
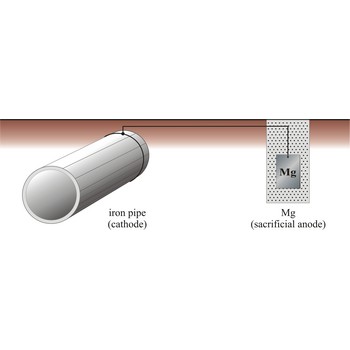
cathodic protection → katodna zaštita
Cathodic protection is a process in which a structural metal, such as iron, is protected from corrosion by connecting it to a metal that has a more negative reduction half-cell potential, which now corrodes instead of iron. There are two major variations of the cathodic method of corrosion protection. The first is called the impressed current method, and the other is called the sacrificial anode method.
copper → bakar
Copper has been known since ancient times. The origin of the name comes from the Latin word cuprum meaning the island of Cyprus famed for its copper mines. It is malleable, ductile, reddish-brown metal. Resistant to air and water. Exposed surfaces form greenish carbonate film. Pure copper occurs rarely in nature. Usually found in sulfides as in chalcopyrite (CuFeS2), coveline (CuS), chalcosine (Cu2S) or oxides like cuprite (Cu2O). Most often used as an electrical conductor. Also used in the manufacture of water pipes. Its alloys are used in jewellery and for coins.
Citing this page:
Generalic, Eni. "Difuzijska struja." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table