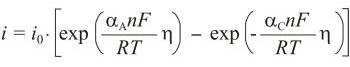Biot-Savart law → Biot-Savartov zakon
The magnetic field B due to a current-carrying conductor can be determined by Biot-Savart law. The contribution to magnetic field set up at distance r by the current element IdL is given by expression:
where μ0 is permeability constant. It plays a role in magnetic problems equivalent to the role of permittivity constant μ0 in electrostatics problems. In order to obtain B, contributions of all current elements have to be integrated. In case of a long straight conductor, carrying current I, Biot-Savart law gives:
SI unit for magnetic field B is tesla (T).
Permaeability constant μ0 has value 4π×10-7 T m A-1.
henry → henri
Henry (H) is the SI derived unit of inductance equal to the inductance of a closed circuit in which an e.m.f. of one volt is produced when the electric current in the circuit varies uniformly at a rate of one ampere per second (H = V·s/A). The unit was named after the American physicst Joseph Henry (1797-1878).
immersion plating → galvaniziranje uranjanjem
Immersion plating involves depositing a metallic coating on a metal immersed in a liquid solution, without the aid of an external electric current. Also called dip plating.
Butler-Volmer equation → Butler-Volmerova jednadžba
Butler-Volmer equation is an activation controlled reaction, the one for which the rate of reaction is controlled solely by the rate of the electrochemical charge transfer process, which is in turn an activation-controlled process. This gives rise to kinetics that are described by the Butler-Volmer equation:
where io is exchange current density, η is overpotential (η = E - Eo), n is number of electrons, αA is anodic transfer coefficient, and αC is cathodic transfer coefficient
cathode → katoda
Cathode is a negative electrode of an electrolytic cell to which positively charged ions (cations) migrate when a current is passed as in electroplating baths.
In a primary or secondary cell (battery or accumulator) the cathode is the electrode that spontaneously becomes negative during discharge, and form which therefore electrons emerge.
In vacuum electronic devices electrons are emitted by the cathode and flow to the anode.
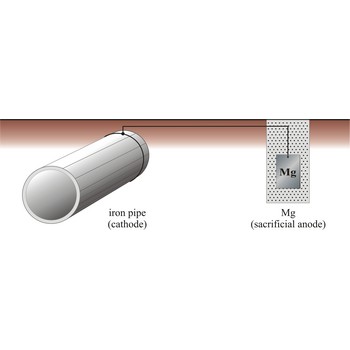
cathodic protection → katodna zaštita
Cathodic protection is a process in which a structural metal, such as iron, is protected from corrosion by connecting it to a metal that has a more negative reduction half-cell potential, which now corrodes instead of iron. There are two major variations of the cathodic method of corrosion protection. The first is called the impressed current method, and the other is called the sacrificial anode method.
convection → konvekcija
Convection is the process by which heat is transferred from one part of a fluid to another by movement of the fluid itself. There are two methods by which this can be carried out.
Natural convection, in which movement occurs as a result of gravity. Heat transferred through a fluid medium, such as air or water, by currents that result from the rising of less dense, warm fluid and the sinking of heavier, cooler fluid.
Forced convection is where hot fluid is transferred from one region to another by a mechanical means (fans or pumps).
impedance → impedancija
Impedance is the analogue of the resistance or resistivity when applied to alternating current. That is, it is a measure of a material’s inability to carry the electrical current. In many materials the impedance varies as the frequency of the applied electrical potential changes, due to the properties of the conducting liquid or solid. In electrochemistry, the impedance of the electrodes is also frequency dependent.
irreversible galvanic cell → nepovrativi galvanski članak
Irreversible galvanic cell is a chemical source of direct current, in which reactions that take place on the electrodes are irreversible.
Lewis number → Lewisova značajka
Lewis number (Le) is a dimensionless quantity used in fluid mechanics, defined by
where a is thermal diffusivity and D is diffusion coefficient.
Citing this page:
Generalic, Eni. "Diffusion current." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table