diastereoisomer → dijastereoizomer
Diastereoisomers (diastereomers) are stereoisomers of a compound having two or more chiral centers that are not a mirror image of another stereoisomer of the same compound. For example, in the structure below, 1 and 2 are enantiomers and so are 3 and 4; 1 and 3 are diastereoisomers, as are 2 and 4. Unlike enantiomers, diastereoisomers need not have closely similar physical and chemical properties
dielectric constant → dielektrična konstanta
Dielectric constant or permittivity (ε) is an index of the ability of a substance to attenuate the transmission of an electrostatic force from one charged body to another. The lower the value, the greater the attenuation. The standard measurement apparatus utilises a vacuum whose dielectric constant is 1. In reference to this, various materials interposed between the charged terminal have the following value at 20 °C:
| vacuum | 1 |
| air | 1.00058 |
| glass | 3 |
| benzene | 2.3 |
| acetic acid | 6.2 |
| ammonia | 15.5 |
| ethanol | 25 |
| glycerol | 56 |
| water | 81 |
The exceptionally high value for water accounts for its unique behaviour as a solvent and in electrolytic solutions. Dielectric constant values decrease as the temperature rises.
diffraction → difrakcija
Diffraction is the ability of a wave to bend around the edges of obstacles or holes. The effect is most noticeable when the obstacle or hole is comparable to the size of the wavelength
dioxin → dioksin
Dioxin is a general term that describes a group of hundreds of chemicals that are highly persistent in the environment. The most toxic compound is 2,3,7,8-tetrachlorodibenzo-p-dioxin or TCDD. The toxicity of other dioxins and chemicals like PCBs that act like dioxin are measured in relation to TCDD. Dioxin is formed as an unintentional by-product of many industrial processes involving chlorine such as waste incineration, chemical and pesticide manufacturing and pulp and paper bleaching. Dioxin was the primary toxic component of Agent Orange, found at Love Canal in Niagara Falls, NY and was the basis for evacuations at Times Beach, MO and Seveso, Italy.
Dioxin is formed by burning chlorine-based chemical compounds with hydrocarbons. The major source of dioxin in the environment comes from waste-burning incinerators of various sorts and also from backyard burn-barrels. Dioxin pollution is also affiliated with paper mills which use chlorine bleaching in their process, with the production of Polyvinyl Chloride (PVC) plastics, and with the production of certain chlorinated chemicals (like many pesticides).
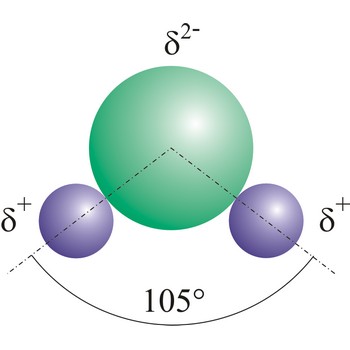
dipole molecule → dipolna molekula
Dipole molecules are created when mutual electronic pair at covalent bond is asymmetrical. If different atoms are bonded by a covalent bond, which can have different electron affinity, then the the atom with greater electron affinity will attract the electron pairs more strongly. In this way an asymmetrical distribution of negative charge appears in a molecule, so one part of the molecule becomes relatively negatively (the one closer to the electron pair) and the other becomes relatively positively charged.
disaccharide → disaharid
Disaccharides are compounds in which two monosaccharides are joined by a glycosidic bond. A glycosidic bond to the anomeric carbon can be either α or β. For example, maltose, the disaccharide obtained by enzyme-catalyzed hydrolysis of starch, consists of two D-glucopyranose units joined by a 1,4’-α-glycoside bond. The "prime" superscript indicates that C-4 is not in the same ring as C-1. Unlike the other disaccharides, sucrose is not a reducing sugar and does not exhibit mutarotation because the glycosidic bond is between the anomeric carbon of glucose and the anomeric carbon of fructose.
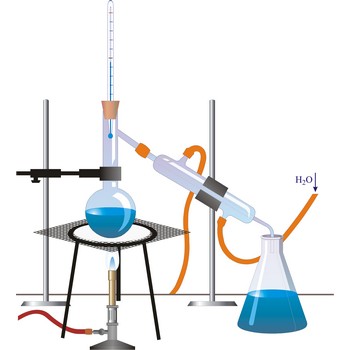
distillation → destilacija
Distillation is a process of boiling a liquid and condensing and collecting the vapour. The liquid collected is the distillate. The usual purpose of distillation is purification or separation of the components of a mixture. This is possible because the composition of the vapour is usually different from that of liquid mixture from which it is obtained. Petrol, kerosene, fuel oil, and lubricating oil are produced from petroleum by distillation.
dropping mercury electrode → kapajuća živina elektroda
Dropping mercury electrode (DME) is a working electrode arrangement for polarography in which mercury continuously drops from a reservoir through a capillary tube (internal diameter 0.03 - 0.05 mm) into the solution. The optimum interval between drops for most analyses is between 2 and 5 s. The unique advantage to the use of the DME is that the constant renewal of the electrode surface, exposed to the test solution, eliminates the effects of electrode poisoning.
dry cell → suhi članak
Dry cell or Leclanche cell is a primary cell having a zinc anode, a carbon (graphite) cathode surrounded by manganese dioxide, and a paste containing ammonium chloride as electrolyte. The electromotive force (emf) produced by a dry cell is 1.5 V. Dry cell is not reversible and therefore have a limited operating life. It is invented by the French engineer Georges Leclanché (1839.-1882.) in 1866.
Citing this page:
Generalic, Eni. "Curtain rod width." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table