superfluid helium → superfluidni helij
Superfluidity in helium-4 was discovered in 1938 by the Soviet physicist Pyotr Leonidovich Kapitsa. Helium-4 exhibits superfluidity when it is cooled below 2.18 K (-270.97 C), which is called the lambda (λ) point. At these temperatures, helium-4 exhibits the characteristics of two distinct fluids, one of which appears to flow without friction. An extensive series of experiments showed that in this state of helium, called helium II (He II), there is an apparent enormous rise in heat conductivity, at an increase rate of about three million. Another unusual property of He II is its mobile, rapid flow through capillaries or over the rim of its containment vessel as a thin film that exhibits no measurable viscosity and appears unaffected by the forces of gravity or evaporation and condensation.
superoxide → superoksid
Superoxides are binary compounds containing oxygen in the -½ oxidation state. Sodium superoxide (NaO2) can be prepared with high oxygen pressures, whereas the superoxides of rubidium, potassium, and cesium can be prepared directly by combustion in air. These compounds are yellow to orange paramagnetic solids. Superoxide ion, O2-, has an unpaired electron, is not particularly stable, and spontaneously decomposes into peroxide over time.
They are strong oxidising agents that vigorously hydrolyze (react with water) to produce superoxide and oxygen gas.
Tafel plot → Tafelov dijagram
Tafel plot is the graph of the logarithm of the current density j against the overpotential η in electrochemistry in the high overpotential limit. An electrode when polarised frequently yields a current potential relationship over a region which can be approximated by:
where η is change in open circuit potential, i is the current density, B and i0 is constants. B is known as the Tafel Slope. If this behaviour is observed a plot of the semilogarithmic components is known as the Tafel line and the diagram is called the Tafel diagram.
terbium → terbij
Terbium was discovered by Carl Gustaf Mosander (Sweden) in 1843. Named after Ytterby, a village in Sweden. It is soft, ductile, silvery-grey, rare earth metal. Oxidizes slowly in air. Reacts with cold water. Terbium is found with other rare earths in monazite sand. Other sources are xenotime and euxenite, both of which are oxide mixtures that can contain up to 1 % terbium. It is used in modest amounts in special lasers and solid-state devices.
thermal expansion → toplinsko rastezanje
Thermal expansion is a change in dimensions of a material resulting from a change in temperature. All objects change size with changes in temperature. The change ΔL in any linear dimension L is given by
in which α is the thermal coefficient of linear expansion, Lo is the initial or reference dimension at temperature To (reference temperature) and ΔT is change in temperature which causes the change in dimension.
The change ΔV in the volume of a sample of solid or liquid is
Here γ is coefficient of volume expansion, Vo is the volume of the sample at temperature To and ΔV is the change in volume over the temperature range ΔT. With isotropic substances, the coefficient of volume expansion can be calculated from the coefficient of linear expansion: γ = 3α.
thermodynamic laws → termodinamički zakoni
Thermodynamic laws are the foundation of the science of thermodynamics:
First law: The internal energy of an isolated system is constant; if energy is supplied to the system in the form of heat dq and work dw, then the change in energy dU = dq + dw.
Second law: No process is possible in which the only result is the transfer of heat from a reservoir and its complete conversion to work.
Third law: The entropy of a perfect crystal approaches zero as the thermodynamic temperature approaches zero.
thermometer → termometar
Thermometers are devices for measuring temperature. Linear and volume thermal expansion are macroscopic properties of matter, which can be easily measured, relative to measurements of microscopic properties, on the basis of which, temperature is defined. Thermometers based on thermal expansion are secondary instruments that is, they have to be calibrated in comparison to a standard thermometer. In a thermometer with liquid, mercury or alcohol is placed in a small glass container. If temperature increases, the liquid undergoes volume expansion and rises in a capillary. The level of the raised liquid is the measure of temperature. Mercury thermometers measure temperatures in the temperature range between -39 °C and 300 °C. Alcohol thermometers measure lower temperatures. Bimetal thermometers have a spiral spring, which consists of two metals with different coefficients of linear expansion. When temperature changes, metals undergo different change in length and the consequence twisting of the spring is transferred to a pointer, the deflection of which is the measure of temperature.
transition metal → prijelazni element
This group of metals is distinguished from other metals not by their physical properties, but by their electronic structure. Transition metals are elements characterized by a partially filled d subshell. The First Transition Series comprises scandium (Sc), titanium (Ti), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni) and copper (Cu). The Second and Third Transition Series include the lanthanides and actinides, respectively.
The transition metals are noted for their variability in oxidation state. Thus, manganese has two electrons in its outside shell and five electrons in the next shell down, and exhibits oxidation states of +1, +2, +3, +4, +5, +6, and +7.
They are also characterised by the fact that well into the series, going from left to right, the properties of the succeeding metals do not differ greatly from the preceding ones.
van der Waals’ equation → van der Waalsova jednadžba
Van der Waals’ equation is an equation of state for real fluids which takes the form:
where p is pressure, Vm is molar volume, T is temperature, R is the molar gas constant, and a and b are characteristic parameters of the substance which describe the effect of attractive and repulsive intermolecular forces.
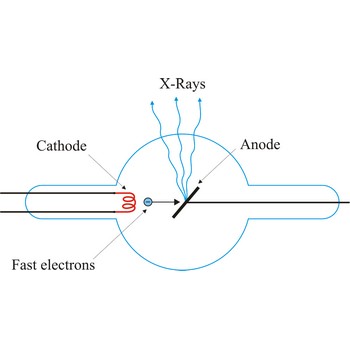
X-ray tube → rendgenska cijev
X-ray tube is a cathode ray tube that focuses energetic streams of electrons on a metal target, causing the metal to emit X-rays. The basic principle of the X-ray tube has not changed significantly since Roentgen's 1895 discovery. Current applied to a metal cathode (about 50 000 V) produces free electrons. The X-rays are produced when the rapidly moving electrons are suddenly stopped as they strike the metal target of the tube.
Citing this page:
Generalic, Eni. "Change of state." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table