octet rule → pravilo okteta
Octet rule states that the chemical properties of the elements repeat on a regular basis with increasing atomic mass, and that the chemical properties of each eight element are similar. Since the inert gases, with the exception of helium have eight electrons in their outer shells, this stable electronic configuration is called the octet rule. In chemical reactions atoms of elements tend to react in such a way as to achieve the electronic configuration of the inert gas nearest to them in the periodic table. There are a number of exceptions to the octet rule.
oxidation number → oksidacijski broj
Atoms can give one or more electrons for bond forming. The valence of any atom, which comes from stechiometrical relation of interbonded atoms, is called an oxidation number or an oxidation degree. Oxidation number of atoms in elementary state is zero. An atom of greater electronegativity has a negative, and an atom of lesser electronegativity has a positive oxidation number.
paper chromatography → papirna kromatografija
Paper chromatography is one of the types of chromatography procedures which runs on a piece of specialized paper. It is a planar chromatography systems wherein a cellulose filter paper acts as a stationary phase on which separation of compounds occurs. The edge of the paper is immersed in a solvent, and the solvent moves up the paper by capillary action.
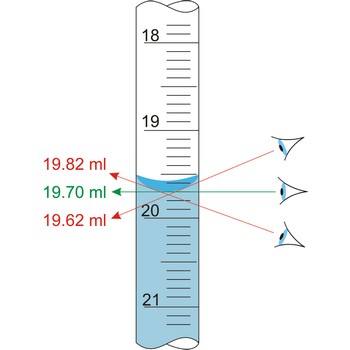
parallax → paralaksa
Parallax is a deceptive change of the position of an object which is observed while the position of the observer changes. Position of eye at all volumetric vessels must be at the same level as the meniscus. If not, the parallax will cause an error while reading the position of the meniscus of a liquid in a burette. It will be a positive mistake if the eye is lower, and negative if the eye is higher than the meniscus plane.
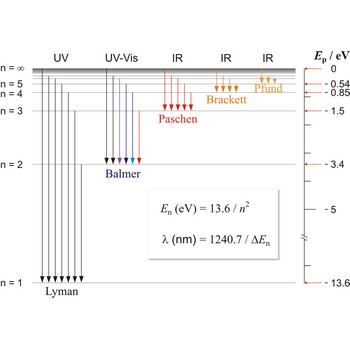
Paschen series → Paschenova serija
Paschen series are the series of lines in the spectrum of the hydrogen atom which corresponds to transitions between the state with principal quantum number n = 3 and successive higher states.
plasma → plazma
Plasma is a highly ionised gas in which the charge of the electrons is balanced by the charge of the positive ions, so that the system as a whole is electrically neutral. Plasmas are created by exposing gases at low pressure to an electric or electromagnetic field. In semiconductor processing, plasmas are used for etching and thin film deposition (the excited state of the gas makes it very reactive). In everyday life plasmas are used to give light in fluorescent light bulbs, neon lamps, and blue insect traps.
polonium → polonij
Polonium was discovered by Marie Curie (Poland) in 1898. Named for Poland, native country of Marie Curie. It is silvery-grey, extremely rare, radioactive metal. Soluble in dilute acids. Highly toxic. Severe radiotoxicity. Carcinogen. Polonium occurs in pitchblende. Produced by bombarding bismuth with neutrons. Used in industrial equipment that eliminates static electricity caused by such processes as rolling paper, wire and sheet metal.
polymorphism → polimorfija
Polymorphism is the ability of a solid substance to crystallise into more than one different crystal structure. Different polymorphs have different arrangements of atoms within the unit cell, and this can have a profound effect on the properties of the final crystallised compound. The change that takes place between crystal structures of the same chemical compound is called polymorphic transformation.
The set of unique crystal structures a given compound may form are called polymorphs. Calcium carbonate is dimorphous (two forms), crystallizing as calcite or aragonite. Titanium dioxide is trimorphous; its three forms are brookite, anatase, and rutile. The prevailing crystal structure depends on both the temperature and the external pressure.
Iron is a metal with polymorphism structure. Each structure stable in the range of temperature, for example, when iron crystallizes at 1 538 °C it is bcc (δ-iron), at 1 394 °C the structure changes to fcc (γ-iron or austenite), and at 912 °C it again becomes bcc (α-iron or ferrite).
Polymorphism of an element is called allotropy.
potential energy → potencijalna energija
Potential energy (Ep) is the energy stored in a body or system as a consequence of its position, shape, or state (this includes gravitation energy, electrical energy, nuclear energy, and chemical energy). Gravitational potential energy is the energy associated with the state of separation between bodies that attracts each other via gravitational force. Elastic potential energy is the energy associated with the state of compression or extension of an elastic object. Thermal energy is associated with the random motions of atoms and molecules in a body.
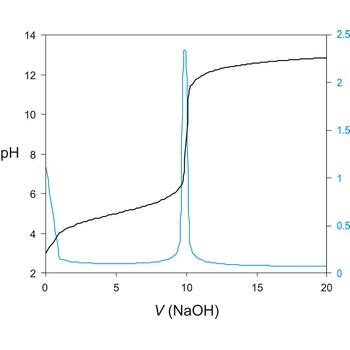
potentiometric titration → potenciometrijska titracija
Potentiometric titration is a volumetric method in which the potential between two electrodes is measured (referent and indicator electrode) as a function of the added reagent volume. Types of potentiometric titrations for the determination of analytes in photoprocessing solutions include acid-base, redox, precipitation, and complexometric.
Potentiometric titrations are preferred to manual titrations, since they are more accurate and precise. They are also more easily adapted to automation, where automated titration systems can process larger volumes of samples with minimal analyst involvement.
A titration curve has a characteristic sigmoid curve. The part of the curve that has the maximum change marks the equivalence point of the titration. The first derivative, ΔE/ΔV, is the slope of the curve, and the endpoint occurs at the volume, V', where ΔE/ΔV has the maximum value.
Citing this page:
Generalic, Eni. "Change of state." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table