van’t Hoff equation → van Hoffova jednadžba
Van’t Hoff equation is the equation expressing the temperature dependence on the equilibrium constant K of a chemical reaction:
where ΔrH° is the standard enthalpy of reaction, R the molar gas constant, and T the temperature.
vanadium → vanadij
Vanadium was discovered by A. M. del Rio (Spain) in 1801 and rediscovered by Nils Sefstrom (Sweden) in 1830. Named after Vanadis, the goddess of beauty in Scandinavian mythology. It is soft, ductile, silvery-white metal. Resistant to corrosion by moisture, air and most acids and alkalis at room temperature. Exposed surfaces form oxide coating. Reacts with concentrated acids. Vanadium is found in the minerals patronite (VS4), vanadinite [Pb5(VO4)3Cl] and carnotite [K2(UO2)2(VO4)2·3H2O]. Pure metal produced by heating with C and Cl to produce VCl3 which is heated with Mg in Ar atmosphere. It is mixed with other metals to make very strong and durable alloys. Vanadium pentoxide (V2O5) is used as a catalyst, dye and fixer-fixer.
volumetric flask → odmjerna tikvica
Volumetric flasks are bottles made of glass, in a pear like in shape with long thin necks and flat bottoms. All come with a ground glass stopper for a tight seal. Volume marking is cut in glass with fluoride acid around the neck, so that parallax should be avoided (flask is put in front of the eyes so that one can see only a straight horizontal line). A volumetric flask is calibrated to contain (TC or In) the indicated volume of water at 20 °C when the bottom of the meniscus is adjusted to just rest on the center of the line marked on the neck of the flask. They are used for preparing the exactly known volume of sample solution and standard solutions of reagents. On each flask with volume designation a temperature on which the flask has been calibrated is designated.
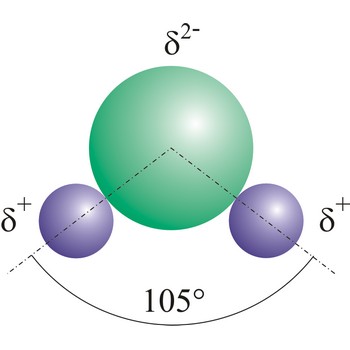
water → voda
Water (H2O) (dihydrogen oxide) is a binary compound that occurs at room temperature as a clear colorless odorless tasteless liquid; freezes into ice below 0 °C and boils above 100 °C. Water is a chemical compound which is essential for living organisms and it is widely used as a solvent.
water gas → vodeni plin
Water gas (blue gas, synthesis gas) is a fuel gas used in industrial synthesis of organic chemicals, and in welding, glassmaking, and other high-temperature industrial applications. Water gas is made by passing steam over a bed of hot coal or coke. It mainly consists of carbon monoxide (CO) and hydrogen (H2), contaminated with small amounts of CO2, N2, CH4, and O2.
Ziegler process → Zieglerov proces
Ziegler process is an industrial process for the manufacture of high-density polyethene using catalysts of titanium(IV) chloride (TiCl4) and aluminium alkyls (e.g. triethylaluminium, Al(C2H5)3). The process was introduced in 1953 by the German chemist Karl Ziegler (1898-1973). It allowed the manufacture of polythene at lower temperatures (about 60 °C) and pressures (about 1 atm) than used in the original process.
water hardness → tvrdoća vode
Hardness is defined as the concentrations of calcium and magnesium ions expressed in terms of calcium carbonate. These minerals in water can cause some everyday problems. They react with soap and produce a deposit called soap curd that remains on the skin and clothes and, because it is insoluble and sticky, cannot be removed by rinsing.
Hard water may also shorten the life of plumbing and water heaters. When water containing calcium carbonate is heated, a hard scale is formed that can plug pipes and coat heating elements. Scale is also a poor heat conductor. With increased deposits on the unit, heat is not transmitted to the water fast enough and overheating of the metal causes failure. Build-up of deposits will also reduce the efficiency of the heating unit, increasing the cost of fuel.
There are two types of water hardness, temporary and permanent.
Temporary Hardness is due to the bicarbonate ion, HCO3-, being present in the water. This type of hardness can be removed by boiling the water to expel the CO2, as indicated by the following equation:
Permanent hardness is due to calcium and magnesium nitrates, sulphates, and chlorides etc. This type of hardness cannot be eliminated by boiling.
| Water supply classification | |
|---|---|
| Hardness | Concentration of Calcium carbonate (mg/L) |
| Soft Water | 0 to 75 |
| Medium Hard Water | 75 to 150 |
| Hard Water | 150 to 300 |
| Very Hard Water | over 300 |
work → rad
Work is the energy required to move an object against an opposing force. Work is usually expressed as a force times a displacement.
When a constant force F acts on a point-like object while the object moves through a displacement s, the force does work W on the object. If force and displacement are at a constant angle Θ to each other, the work is expressed by the scalar product of these two vectors:
When the force F on a point-like object is not constant that is, it depends on the position of the object, the work done by force while object moves from initial position with coordinates (xi, yi, zi) to final position with coordinates (xf, yf, zf)is given by expression:
Where Fx, Fy and Fz are scalar components of the force.
SI unit for work is joule (J); 1 J = 1 Nm = 1 kg m2 s-2. The electron-volt (eV) is commonly used in atomic and nuclear physics.
Heyrovsky-Ilkovic equation → Heyrovsky-Ilkovičeva jednadžba
The Heyrovsky-Ilkovic equation describes the entire current-potential curve (polarographic wave) of a reversible redox system in polarography
where R is the gas constant, T is the absolute temperature, F is the Faraday constant, n denotes the number of electrons taking part in the electrode reaction. E1/2 is a unique potential (for a given reaction and supporting electrolyte) termed the half-wave potential.
In order to obtain E1/2 from the above equation, we plot a graph of ln[(id-i)/i] against E. The intercept on the x-axis gives then an accurate value of E1/2. The slope of the obtained straight line is equal to nF/RT from which n is determined.
Citing this page:
Generalic, Eni. "Celsius temperature scale." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table