thermal expansion → toplinsko rastezanje
Thermal expansion is a change in dimensions of a material resulting from a change in temperature. All objects change size with changes in temperature. The change ΔL in any linear dimension L is given by
in which α is the thermal coefficient of linear expansion, Lo is the initial or reference dimension at temperature To (reference temperature) and ΔT is change in temperature which causes the change in dimension.
The change ΔV in the volume of a sample of solid or liquid is
Here γ is coefficient of volume expansion, Vo is the volume of the sample at temperature To and ΔV is the change in volume over the temperature range ΔT. With isotropic substances, the coefficient of volume expansion can be calculated from the coefficient of linear expansion: γ = 3α.
thermal resistance → toplinski otpor
Heat always flows from a higher to a lower temperature level. The driving force for the heat flux lies in the temperature difference ΔT between two temperature levels. Analogous to Ohm’s law, the following holds:
where H = dQ/dt is heat flux, measured in watts, ΔT is temperature difference across the thermal resistance, measured in kelvin, and Rth is thermal resistance, measured in K/W.
For example, suppose there were two houses with walls of equal thickness; one is made of glass and the other of asbestos. On a cold day, heat would pass through the glass house much faster. The thermal restistance of asbestos is then higher than of glass.
If the thermal Ohm’s law is divided by the heat capacity C, Newton’s law of cooling is obtained:
where dT/dt is rate of cooling or heating, measured in K s-1, and C is heat capacity, measured in J K-1.
thermite → termit
Thermite is a stoichiometric powdered mixture of iron(II) oxide and aluminium for the reaction
The reaction is highly exothermic and the increase in temperature (over 2500 °C) is sufficient to melt the iron produced. It has been used for localized welding of steel object (e.g. railway lines) in the thermit process. Thermite is also used in incendiary bombs.
thermodynamic laws → termodinamički zakoni
Thermodynamic laws are the foundation of the science of thermodynamics:
First law: The internal energy of an isolated system is constant; if energy is supplied to the system in the form of heat dq and work dw, then the change in energy dU = dq + dw.
Second law: No process is possible in which the only result is the transfer of heat from a reservoir and its complete conversion to work.
Third law: The entropy of a perfect crystal approaches zero as the thermodynamic temperature approaches zero.
thermometer → termometar
Thermometers are devices for measuring temperature. Linear and volume thermal expansion are macroscopic properties of matter, which can be easily measured, relative to measurements of microscopic properties, on the basis of which, temperature is defined. Thermometers based on thermal expansion are secondary instruments that is, they have to be calibrated in comparison to a standard thermometer. In a thermometer with liquid, mercury or alcohol is placed in a small glass container. If temperature increases, the liquid undergoes volume expansion and rises in a capillary. The level of the raised liquid is the measure of temperature. Mercury thermometers measure temperatures in the temperature range between -39 °C and 300 °C. Alcohol thermometers measure lower temperatures. Bimetal thermometers have a spiral spring, which consists of two metals with different coefficients of linear expansion. When temperature changes, metals undergo different change in length and the consequence twisting of the spring is transferred to a pointer, the deflection of which is the measure of temperature.
thermosphere → termosfera
Thermosphere is the layer of the earth’s atmosphere extending from the top of the mesosphere (80 km - 90 km above the surface) to about 500 km. It is characterised by a rapid increase in temperature with increasing altitude up to about 200 km, followed by a levelling off in the 300 km - 500 km region.
troposphere → troposfera
Troposphere is the lowest part of the earth’s atmosphere, extending to 10 km to 15 km above the surface. It is characterized by a decrease in temperature with increasing altitude. The exact height varies with latitude and season.
unequal-arm balance → vaga s različitim krakovima
The lever principle on which these scales are constructed is based on the law of physics that at equilibrium the force applied at one end of the lever multiplied by the length of the arm (distance from the fulcrum to the point where the force is applied) must be equal to the product of the force acting at the opposite end of the lever and the length of the other arm.
The unequal-arm balance is preferred for work when large amounts are to be weighed.
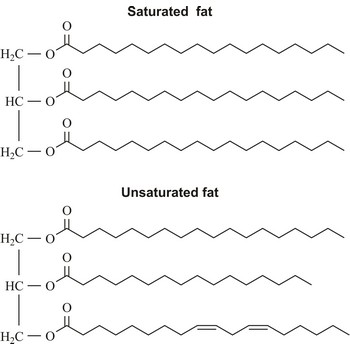
unsaturated fat → nezasićena mast
Unsaturated fats, which include one or more unsaturated fatty acid, are liquid at room temperature (oil) and come from plant oils such as olive, peanut, corn, sunflower, safflower, and soybean. The fish oils may also be high in unsaturated fatty acids.
van der Waals’ equation → van der Waalsova jednadžba
Van der Waals’ equation is an equation of state for real fluids which takes the form:
where p is pressure, Vm is molar volume, T is temperature, R is the molar gas constant, and a and b are characteristic parameters of the substance which describe the effect of attractive and repulsive intermolecular forces.
Citing this page:
Generalic, Eni. "Celsius temperature scale." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table