salt fog test → ispitivanje u slanoj komori
Salt fog test is an accelerated corrosion test in which specimens are exposed to a fine mist of a solution usually containing sodium chloride (typically 5 %). Other contaminants can be added according to desired conditions. It is mainly used to determine the effectiveness of material finishes and protective coatings on materials. Salt-fog testing is also used to determine the effects of salt deposits on the electrical functions of electronic assemblies.
salinity → salinitet
Salinity (S) is a measure of the quantity of dissolved salts in seawater. It is formally defined as the total amount of dissolved solids in seawater in parts per thousand (‰) by weight when all the carbonate has been converted to oxide, the bromide and iodide to chloride, and all organic matter is completely oxidized.
Chlorinity is the oldest of the salinity measures considered and is still a corner-stone in the study of dissolved material in seawater. Based on the principle of constant relative proportions it provides a measure of the total amount of dissolved material in seawater in terms of the concentration of halides. The relationship between chlorinity (Cl) and salinity as set forth in Knudsen’s tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
Practical Salinity (SP) was introduced as a replacement for Chlorinity. Practical Salinity is is relatively easy to measure using standard conductometers, measurements are more precise and less time consuming than measurements of Chlorinity and accurate measurements can even be made in situ. Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution).
Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". For most purposes one can assume that the psu and the ‰, are synonymous.
The global average salinity of ocean waters is about 35 ‰, that is, about 35 g of solid substances are dissolved in 1 kg of seawater.
sedimentary rocks → sedimentne stijene
Sedimentary Rocks are formed by the accumulation and subsequent consolidation of sediments into various types of rock. There are three major types of sedimentary rocks:
Biogenic sedimentary rocks are formed from organic processes when organisms use materials dissolved in water to build a shell or other skeletal structure.
Clastic sedimentary rocks are composed directly of the sediments or fragments from other rocks.
Chemical sedimentary rocks are formed through evaporation of a chemical rich solution.
Based on their sizes, sediment particles are classified, based on their size, into six general categories:
- boulder (>256 mm)
- cobble (64 - 256 mm)
- gravel (2 - 64 mm)
- sand (1/16 - 2 mm)
- silt (1/256 - 1/16 mm)
- clay (<1/256 mm)
sedimentation → sedimentiranje
Sedimentation is a process of separating specifically heavier, suspended matter, than the solution is. Solid matter settles on the bottom of the vessel and the liquid above it is poured off. The settling zone is the largest portion of the sedimentation basin. This zone provides the calm area necessary for the suspended particles to settle. The sludge zone, located at the bottom of the tank, provides a storage area for the sludge before it is removed for additional treatment or disposal.
silver coulometer → srebrni kulometar
Silver coulometer consists of a platinum vessel which acts as a cathode and contains a solution of pure silver nitrate as an electrolyte (c(AgNO3) = 1 mol/L). A rod of pure silver enclosed in a porous pot acts as the anode. The current density at the anode should not exceed 0.2 Acm-2. After electrolysis, the electrolyte is taken out and the platinum vessel is washed, dried and weighed. The increase in the weight gives the amount of silver deposited (96500 C of electricity deposits 107.88 g of silver). From the mass of the silver deposited, the coulomb involved in the reaction can be calculated.
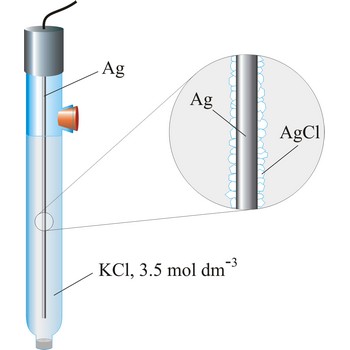
silver/silver-chloride electrode → srebro/srebrov klorid elektroda
Silver/silver-chloride electrode is by far the most common reference type used today because it is simple, inexpensive, very stable and non-toxic. It is mainly used with saturated potassium chloride electrolyte, but can be used with lower concentrations such as 3.5 mol dm-3 or 1 mol dm-3 potassium chloride. Silver/silver-chloride electrode is a referent electrode based on the following halfreaction
| Potential vs. SHE / V | ||
|---|---|---|
| t / °C | 3.5 mol dm-3 | sat. solution |
| 15 | 0.212 | 0.209 |
| 20 | 0.208 | 0.204 |
| 25 | 0.205 | 0.199 |
| 30 | 0.201 | 0.194 |
| 35 | 0.197 | 0.189 |
sol → sol
Sols are dispersions of small solid particles in a liquid. The particles may be macromolecules or may be clusters of small molecules. Lyophobic sols are those in which there is no affinity between the dispersed phase and the liquid (e.g. silver chloride dispersed in water). Lyophobic sols are inherently unstable, in time the particles aggregate, and form a precipitate. Lyiophilic sols, on the other hand, are more like true solutions in which the solute molecules are large and have an affinity for the solvent (e.g. starch in water). Association colloids are systems in which the dispersed phase consists of clusters of molecules that have lyophobic and lyophilic parts (e.g. soap in water).
solubility → topljivost
Solubility is the maximum amount of solute that dissolves in a given quantity of solvent at a specific temperature. Generally, for a solid in a liquid, solubility increases with temperature; for a gas, solubility decreases. Common measures of solubility include the mass of solute per unit mass of solution (mass fraction), mole fraction of solute, molality, molarity, and others.
solubility product constant → konstanta produkta topljivosti
Solubility product constant (Ksp) (or the solubility product) is the product of the molar concentrations of the constituent ions, each raised to the power of its stoichiometric coefficient in the equilibrium equation. For instance, if a compound AaBb is in equilibrium with its solution
the solubility product is given by
spectrophotometer → spektrofotometar
Spectrophotometer is an instrument for measuring the amount of light absorbed by a sample.
The absorption of light by a substance in a solution can be described mathematically by the Beer-Lambert law
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
Citing this page:
Generalic, Eni. "Binary solution." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table